Journal of Catalysis ( IF 7.3 ) Pub Date : 2020-09-25 , DOI: 10.1016/j.jcat.2020.09.016 Prakash Natarajan , Hassnain Abbas Khan , Ahsan Jaleel , Dae Sung Park , Dong-Chang Kang , Sungho Yoon , Kwang-Deog Jung
|
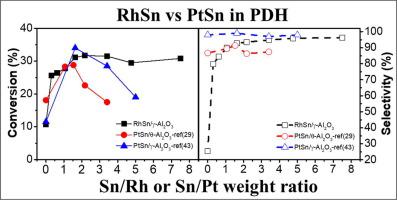
The pronounced effect of Sn on RhSn/γ-Al2O3 catalysts for propane dehydrogenation (PDH) is studied. A 0.5- wt% Rh/γ-Al2O3 catalyst (Rh(0.5)) without Sn exhibits very low propane (C3) conversion (10.7%) and low selectivity (25.4%) for propylene (C3=) at 600 °C and a WHSV of 10.8 h−1. However, the Sn addition to Rh(0.5) increases both the C3 conversion and the C3= yield. Finally, the Rh(0.5)Sn(3.0) catalyst achieves a C3 conversion of 30.8% and a C3= selectivity of 96.3%. It has been reported that PtSn catalysts show an optimized C3= at the Sn/Pt molar ratio of about 1.0, increasing the propene selectivity at the expense of catalytic activity. This is not the case for the RhSn system. The primary product of Rh(0.5) is methane (65%) at the initial time and severe coke formation covers the exposed Rh metal, resulting in low C3 conversion. Both the C3 conversion and the C3= yield increase with increasing Sn content. The low C3 conversion and C3= yield of Rh(0.5) are ascribed to cracking via the strong adsorption of intermediates during propane dehydrogenation, resulting in severe coke formation. The main role of Sn on Rh(0.5) in propane dehydrogenation is to suppress cracking of strongly adsorbed intermediates during dehydrogenation; the gain in C3= selectivity outweighs the loss of activity for propane dehydrogenation to propylene up to a high Sn addition of 3 wt%.
中文翻译:

锡对RhSn丙烷脱氢催化剂的显著作用
的Sn对RhSn /γ-Al系的显着的效果2 ö 3催化剂用于丙烷脱氢(PDH)进行了研究。甲0.5-重量%的Rh /γ-Al系2 ö 3催化剂铑(Rh(0.5)),而不表现出的Sn非常低的丙烷(C 3)转化率(10.7%)和低的选择性(25.4%)为丙烯(C 3 =)在600°C,WHSV为10.8 h -1。但是,向Rh(0.5)中添加Sn可以提高C 3转化率和C 3 =产率。最后,Rh(0.5)Sn(3.0)催化剂的C 3转化率为30.8%,C 3 =选择性为96.3%。据报道,PtSn催化剂在约1.0的Sn / Pt摩尔比下显示出优化的C 3 =,以牺牲催化活性为代价提高了丙烯的选择性。RhSn系统不是这种情况。Rh(0.5)的主要产物在初始时间为甲烷(65%),严重的焦炭形成覆盖了暴露的Rh金属,导致低C 3转化率。C 3转化率和C 3 =产率都随Sn含量的增加而增加。低C 3转换和C 3 =Rh(0.5)的收率归因于丙烷脱氢过程中中间体的强烈吸附导致裂化,从而导致严重的焦炭形成。丙烷脱氢中Sn对Rh(0.5)的主要作用是抑制脱氢过程中强吸附中间体的裂解。在高达3 wt%的高Sn添加量下,C 3 =选择性的增加超过了丙烷脱氢制丙烯的活性的损失。



























 京公网安备 11010802027423号
京公网安备 11010802027423号