Letters in Organic Chemistry ( IF 0.8 ) Pub Date : 2020-10-31 , DOI: 10.2174/1570178617666200224103813 Somayeh Mirdoraghi 1 , Hamed Douroudgari 1 , Farideh Piri 1 , Morteza Vahedpour 1
|
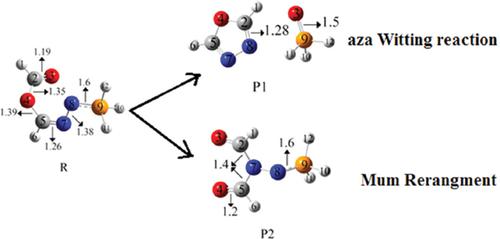
For (Z)-(Z)-N-(λ5-phosphanylidene) formohydrazonic formic anhydride, Aza-Wittig reaction and Mumm rearrangement are studied using both density functional and coupled cluster theories. For this purpose, two different products starting from one substrate are considered that are competing with each other. The obtained products, P1 and P2, are thermodynamically favorable. The product of the aza-Wittig reaction, P1, is more stable than the product of Mumm rearrangement (P2). For the mentioned products, just one reliable pathway is separately proposed based on unimolecular reaction. Therefore, the rate constants based on RRKM theory in 300-600 K temperature range are calculated. Results show that the P1 generation pathway is a suitable path due to low energy barriers than the path P2. The first path has three steps with three transition states, TS1, TS2, and TS3. The P2 production path is a single-step reaction. In CCSD level, the computed barrier energies are 14.55, 2.196, and 10.67 kcal/mol for Aza-Wittig reaction and 42.41 kcal/mol for Mumm rearrangement in comparison with the corresponding complexes or reactants. For final products, the results of the computational study are in a good agreement with experimental predictions.
中文翻译:

(Z)-(Z)-N-(λ5-膦酰基亚甲基)甲酸肼基甲酸酐的单分子反应动力学和机理的计算研究:分子内氮杂-维蒂希反应与木乃伊重排竞争
对于(Z)-(Z)-N-(λ5-膦亚基)甲酸肼基甲酸酐,使用密度泛函和耦合簇理论研究了Aza-Wittig反应和Mumm重排。为此,考虑从一种基材开始的两种不同的产品彼此竞争。所得产物P1和P2在热力学上是有利的。氮杂-维蒂希反应的产物P1比Mumm重排产物(P2)更稳定。对于上述产品,仅基于单分子反应提出了一种可靠的途径。因此,基于RRKM理论计算了300-600 K温度范围内的速率常数。结果表明,由于路径P2的能垒低,P1生成路径是一条合适的路径。第一条路径包含三个步骤,其中包含三个过渡状态TS1,TS2,和TS3。P2的生产路径是一步反应。与相应的配合物或反应物相比,在CCSD水平上,对于Aza-Wittig反应,计算出的势垒能量为14.55、2.196和10.67 kcal / mol,对于Mumm重排,计算出的势垒能量为42.41 kcal / mol。对于最终产品,计算研究的结果与实验预测非常吻合。



























 京公网安备 11010802027423号
京公网安备 11010802027423号