Medicinal Chemistry ( IF 2.3 ) Pub Date : 2021-06-30 , DOI: 10.2174/1573406416666200403075826 Alana de Vasconcelos 1 , Ana Júlia Zulian Boeira 1 , Bruna Bento Drawanz 1 , Nathalia Stark Pedra 2 , Natália Pontes Bona 2 , Francieli Moro Stefanello 2 , Wilson Cunico 1
|
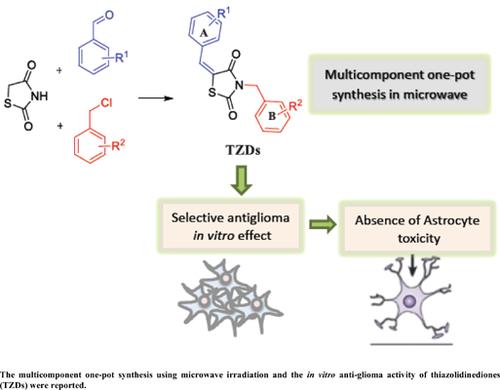
Background: Thiazolidinediones (TZDs) represent an important class of heterocyclic compounds that have versatile biological activities, including anticancer activity. Glioma is one of the most common primary brain tumors, and it is responsible for most of the deaths caused by primary brain tumors. In the present work, 2,4-thiazolidinediones were synthesized via a multicomponent microwave one-pot procedure. The cytotoxicity of compounds was analyzed in vitro using rat (C6) and mouse (GL261) glioblastoma cell lines and primary cultures of astrocytes.
Objective: This study aims to synthesize and characterize 2,4-thiazolidinediones and evaluate their antitumor activity.
Methods: TZDs were synthesized from three components: 2,4-thiazolidinedione, arene-aldehydes, and aryl chlorides. The reactions were carried out inside a microwave and monitored using thinlayer chromatography (TLC). Compounds were identified and characterized using gas chromatography coupled to mass spectrometry (CG-MS) and hydrogen (1H-NMR) and carbon nuclear magnetic resonance spectroscopy (13C-NMR). The antitumor activity was analyzed using the 3-(4,5- dimethyl)-2,5-diphenyltetrazolium bromide (MTT) reduction test, in which cell viability was verified in the primary cultures of astrocytes and in rat and mouse glioblastoma cells exposed to the synthesized compounds. The cytotoxicity of all derivatives was analyzed at the 100 μM concentration, both in astrocytes and in the mouse and rat glioblastoma cell lines. The compounds that showed the best results, 4CI and 4DI, were also tested at concentrations 25, 50, 100, 175, and 250 μM to obtain the IC50.
Results: Seventeen TZD derivatives were easily obtained through one-pot reactions in 40 minutes with yields ranging from 12% to 49%. All compounds were cytotoxic to both glioblastoma cell lines without being toxic to the astrocyte primary cell line at 100 μM, thus demonstrating a selective activity. Compounds 4CI and 4DI showed the best results in the C6 cells: IC50 of 28.51 μM and 54.26 μM, respectively.
Conclusion: The compounds were not cytotoxic in astrocyte culture, demonstrating selectivity for malignant cells. Changes in both rings are important for anti-glioma activity in the cell lines tested. TZD 4CI had the best anti-glioma activity.
中文翻译:

2,4-噻唑烷二酮作为前体在 C6 和 GL261 细胞中合成具有抗神经胶质瘤活性的化合物
背景:噻唑烷二酮 (TZD) 是一类重要的杂环化合物,具有多种生物活性,包括抗癌活性。胶质瘤是最常见的原发性脑肿瘤之一,它是导致原发性脑肿瘤死亡的主要原因。在目前的工作中,2,4-噻唑烷二酮是通过多组分微波一锅法合成的。使用大鼠 (C6) 和小鼠 (GL261) 胶质母细胞瘤细胞系和星形胶质细胞的原代培养物在体外分析了化合物的细胞毒性。
目的:本研究旨在合成和表征 2,4-噻唑烷二酮并评估其抗肿瘤活性。
方法:TZDs 由三种成分合成:2,4-噻唑烷二酮、芳烃醛和芳基氯。反应在微波内进行,并使用薄层色谱 (TLC) 进行监测。使用气相色谱-质谱联用 (CG-MS) 和氢 ( 1 H-NMR) 和碳核磁共振波谱 ( 13核磁共振)。使用 3-(4,5-二甲基)-2,5-二苯基溴化四唑 (MTT) 还原试验分析抗肿瘤活性,其中在星形胶质细胞的原代培养物以及暴露于合成的化合物。在 100 μM 浓度下分析了所有衍生物在星形胶质细胞以及小鼠和大鼠胶质母细胞瘤细胞系中的细胞毒性。显示最佳结果的化合物 4CI 和 4DI 也在 25、50、100、175 和 250 μM 的浓度下进行测试,以获得 IC 50。
结果:在40分钟内通过一锅反应轻松获得17种TZD衍生物,产率范围为12%至49%。所有化合物在 100 μM 时对两种胶质母细胞瘤细胞系都具有细胞毒性,而对星形胶质细胞原代细胞系没有毒性,因此显示出选择性活性。化合物 4CI 和 4DI 在 C6 细胞中显示出最好的结果:IC 50 分别为 28.51 μM 和 54.26 μM。
结论:这些化合物在星形胶质细胞培养物中没有细胞毒性,显示出对恶性细胞的选择性。两个环的变化对于测试细胞系的抗神经胶质瘤活性很重要。TZD 4CI 具有最好的抗胶质瘤活性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号