Medicinal Chemistry ( IF 2.3 ) Pub Date : 2020-10-31 , DOI: 10.2174/1573406415666190826162339 Luis D. Pedro-Hernández 1 , Ulises Organista-Mateos 1 , Luis Isaac Allende-Alarcón 1 , Elena Martínez-Klimova 2 , Teresa Ramírez-Ápan 1 , Marcos Martínez-García 1
|
Background: One of the possible ways of improving the activity and selectivity profile of anticancer agents is to design drug carrier systems employing nanomolecules. Calix[4]arene derivatives and chlorambucil and ibuprofen are important compounds that exhibit interesting anticancer properties.
Objective: The objective of this article is the synthesis of new calix[4]arene-derivative conjugates of chlorambucil or ibuprofen with potential anticancer activity.
Methods: Cytotoxicity assays were determined using the protein-binding dye sulforhodamine B (SRB) in microculture to measure cell growth as described [19, 20]. Conjugates of chlorambucil and resorcinarene-dendrimers were prepared in 2% DMSO and added into the culture medium immediately before use. Control cells were treated with 2% DMSO.
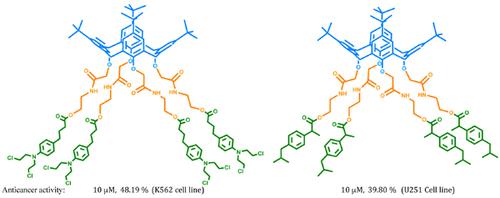
Results: Thus, calix[4]arene-derivative conjugates of chlorambucil or ibuprofen showed good stability of the chemical link between drug and spacer. Evaluation of the cytotoxicity of the calix[4]arene chlorambucil or ibuprofen conjugates employing a sulforhodamine B (SRB) assay in K-562 (human chronic myelogenous leukemia cells) and U-251 (human glioblastoma cells) demonstrated that the conjugate was more potent as an antiproliferative agent than free chlorambucil and ibuprofen. The conjugates did not show any activity against the COS-7 African green monkey kidney fibroblast cell line.
Conclusion: In the paper, we report the synthesis and spectroscopic analyses of new calix[4]arene derivative conjugates of chlorambucil or ibuprofen. Cytotoxicity assays revealed that at 10 μM, the conjugates were very active against K-562 (human chronic myelogenous leukemia cells) and U- 251 (human glioblastoma cells) cancer cells' proliferation. In order to explain the molecular mechanisms involved in the anticancer activity of calix[4]arene chlorambucil or ibuprofen conjugates, our research will be continued.
中文翻译:

杯[4]芳烃缀合物改善氯丁酸丁苯和布洛芬的抗癌活性
背景:改善抗癌药活性和选择性的一种可能方法是设计使用纳米分子的药物载体系统。杯[4]芳烃衍生物和苯丁酸氮芥和布洛芬是重要的化合物,显示出令人感兴趣的抗癌特性。
目的:本文的目的是合成苯丁酸氮芥或布洛芬具有潜在抗癌活性的新杯[4]芳烃-衍生物。
方法:按照文献[19,20]所述,在微培养中使用蛋白结合染料磺基罗丹明B(SRB)测定细胞毒性,以测量细胞生长。在2%DMSO中制备苯丁酸氮芥和间苯二甲烯树状大分子的结合物,并在使用前立即添加到培养基中。对照细胞用2%DMSO处理。
结果:因此,苯丁酸氮芥或布洛芬的杯[4]芳烃-衍生物缀合物显示药物与间隔基之间的化学连接具有良好的稳定性。在K-562(人类慢性粒细胞白血病细胞)和U-251(人类胶质母细胞瘤细胞)中使用磺基罗丹明B(SRB)分析评估杯[4]芳烃苯丁酸氮芥或布洛芬偶联物的细胞毒性,结果表明该偶联物更有效作为抗增殖剂,比游离苯丁酸氮芥和布洛芬更有效。结合物对COS-7非洲绿猴肾成纤维细胞系没有任何活性。
结论:在本文中,我们报告了苯丁酸氮芥或布洛芬的新杯[4]芳烃衍生物共轭物的合成和光谱分析。细胞毒性试验表明,偶联物在10μM时对K-562(人类慢性骨髓性白血病细胞)和U-251(人类胶质母细胞瘤细胞)癌细胞的增殖非常活跃。为了解释杯[4]芳烃苯丁酸氮芥或布洛芬偶联物的抗癌活性的分子机制,我们将继续研究。


























 京公网安备 11010802027423号
京公网安备 11010802027423号