Medicinal Chemistry ( IF 2.3 ) Pub Date : 2021-05-31 , DOI: 10.2174/1573406415666191107121757 Ahmed H. Ismail 1 , Ahmed M. Abdula 2 , Ivan H.R. Tomi 2 , Ali H.R. Al-Daraji 2 , Younis Baqi 1
|
Background: The frequent use of antibacterial agents leads to antimicrobial resistance, which is one of the biggest threats to global health today. Therefore, the discovery of novel antimicrobial agents is still urgently needed to overcome the severe infections caused by these putative pathogens resistant to currently available drugs.
Objective: The present work was aimed to synthesize and investigate the preliminary structureactivity relationships (SARs) of isoxazoline and pyrazoline derivatives as antimicrobial agent.
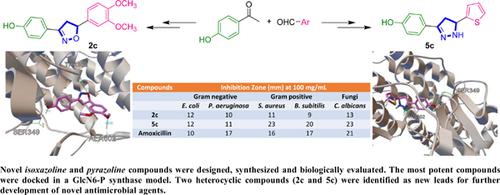
Methods: Target compounds were obtained in a multistep reaction synthesis and the antimicrobial activity was investigated in several species; two-gram negative (Escherichia coli and Pseudomonas aeruginosa), two-gram positive (Staphylococcus aureus and Bacillus subtilis) and one fungi (Candida albicans), using cup-plate agar diffusion method. The most potent compounds were docked into glucosamine-6-phosphate synthase (GlcN-6-P), the molecular target enzyme for antimicrobial agents, using Autodock 4.2 program.
Results: Herein, thirteen novel target compounds were synthesized in moderate to good isolated yield. Based on the SARs, two compounds (2c and 5c) were found to be potent antimicrobial agents on all tested targets, recording potency higher than amoxicillin, the standard antimicrobial drug. Compound 2b identified as selective for gram-negative, while compound 7a found to be selective for gram-positive. The hit compounds (2c, 5a, 5c and 5d) were subjected to a docking study on glucosamine-6-phosphate synthase (GlcN-6-P). All hits were found to bind to the orthosteric (active) site of the enzyme, which might represent a competitive mechanism of inhibition.
Conclusion: The newly synthesized heterocyclic compounds could serve as potent leads for the development of novel antimicrobial agents.
中文翻译:

新型3,5-二取代-2-异恶唑啉和1,3,5-三取代-2-吡唑啉衍生物的合成,抗菌评价和对接研究
背景:频繁使用抗菌剂会导致抗菌素耐药性,这是当今对全球健康的最大威胁之一。因此,仍然迫切需要发现新的抗微生物剂,以克服由这些对当前可用药物具有抗性的推定病原体引起的严重感染。
目的:本工作旨在合成和研究异恶唑啉和吡唑啉衍生物作为抗菌剂的初步结构活性关系(SAR)。
方法:通过多步反应合成获得目标化合物,并研究了几种菌种的抗菌活性。使用杯板琼脂扩散法,两克阴性(大肠埃希氏菌和铜绿假单胞菌),两克阳性(金黄色葡萄球菌和枯草芽孢杆菌)和一种真菌(白色念珠菌)。使用Autodock 4.2程序,将最有效的化合物对接至氨基葡萄糖6-磷酸合酶(GlcN-6-P),这是抗菌剂的分子靶标酶。
结果:在本文中,以中等至良好的分离收率合成了13种新型目标化合物。基于SAR,发现两种化合物(2c和5c)是对所有测试目标均有效的抗菌剂,记录的效力高于标准抗菌药物阿莫西林。化合物2b被鉴定为对革兰氏阴性菌具有选择性,而化合物7a被发现对革兰氏阳性菌具有选择性。对命中的化合物(2c,5a,5c和5d)进行了氨基葡萄糖-6-磷酸合酶(GlcN-6-P)的对接研究。发现所有命中都结合到酶的正构(活性)位点,这可能代表抑制的竞争机制。
结论:新合成的杂环化合物可作为开发新型抗菌剂的有效线索。


























 京公网安备 11010802027423号
京公网安备 11010802027423号