Medicinal Chemistry ( IF 2.3 ) Pub Date : 2021-05-31 , DOI: 10.2174/1573406415666191206095032 Florentinus D O Riswanto 1 , Mira S A Rawa 2 , Vikneswaran Murugaiyah 2 , Nurul H Salin 3 , Enade P Istyastono 1 , Maywan Hariono 1 , Habibah A Wahab 2
|
Background: Chalcones, originated from natural product, have been broadly studied their biological activity against various proteins which at the molecular level, are responsible for the progress of the diseases in cancer (e.g. kinases), inflammation (oxidoreductases), atherosclerosis (cathepsins receptor), and diabetes (e.g. α-glucosidase).
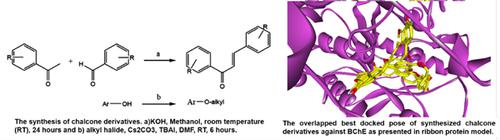
Objective: Here we synthesize 10 chalcone derivatives to be evaluated their in vitro enzymatic inhibition activity against acetylcholinesterase (AChE) and butyrylcholinesterase (BChE).
Methods: The synthesis was carried out using Claissen-Schimdt condensation and the in vitro assay was conducted using Ellman Method.
Results: Compounds 2b and 4b demonstrated as the best IC50 of 9.3 μM and 68.7 μM respectively, towards AChE and BChE inhibition. Molecular docking studies predicted that this activity might be due to the interaction of the chalcones with important amino acid residues in the binding site of AChE such as SER200 and in that of BChE, such as TRP82, SER198, TRP430, TYR440, LEU286 and VAL288.
Conclusion: Chalcone can be used as the scaffold for cholinesterase inhibitor, in particularly either fluorine or nitro group to be augmented at the para-position of Ring B, whereas the hydrophobic chain is necessary at the meta-position of Ring B.
中文翻译:

查尔酮衍生物的抗胆碱酯酶活性:合成、体外测定和分子对接研究
背景:查尔酮来源于天然产物,已经广泛研究了它们对各种蛋白质的生物活性,这些蛋白质在分子水平上是导致癌症(例如激酶)、炎症(氧化还原酶)、动脉粥样硬化(组织蛋白酶受体)等疾病进展的原因和糖尿病(例如α-葡萄糖苷酶)。
目的:在这里我们合成了 10 种查尔酮衍生物,以评估它们对乙酰胆碱酯酶 (AChE) 和丁酰胆碱酯酶 (BChE) 的体外酶抑制活性。
方法:使用克莱森-施姆特缩合进行合成,并使用埃尔曼方法进行体外测定。
结果: 化合物 2b 和 4b 证明对 AChE 和 BChE 抑制的最佳 IC 50 分别为 9.3 μM 和 68.7 μM。分子对接研究预测,这种活性可能是由于查耳酮与 AChE 结合位点(如 SER200)和 BChE(如 TRP82、SER198、TRP430、TYR440、LEU286 和 VAL288)结合位点中重要氨基酸残基的相互作用。
结论:查尔酮可作为胆碱酯酶抑制剂的支架,特别是在B环的对位增加氟或硝基,而在B环的间位需要疏水链。


























 京公网安备 11010802027423号
京公网安备 11010802027423号