Medicinal Chemistry ( IF 2.3 ) Pub Date : 2020-10-31 , DOI: 10.2174/1573406415666190724145158 Beatriz C. Marques 1 , Mariana B. Santos 1 , Daiane B. Anselmo 1 , Diego A. Monteiro 2 , Eleni Gomes 2 , Marilia F.C. Saiki 2 , Paula Rahal 2 , Pedro L. Rosalen 3 , Janaina C.O. Sardi 3 , Luis O. Regasini 1
|
Background: Chalcones substituted by methoxyl groups have presented a broad spectrum of bioactivities, including antifungal, antibacterial and antiproliferative effects. However, a clear and unambiguous investigation about the relevance of this substituent on the chalcone framework has not been described.
Objective: The purpose of this work is to assess the antibacterial, antifungal and antiproliferative activities of the two series of seventeen synthesized regioisomeric methoxychalcones. Series I and II were constituted by chalcones substituted by methoxyl groups on rings A (5–12) and B (13–21), respectively. In addition, the library of methoxychalcones was submitted to in silico drug-likeness and pharmacokinetics properties predictions.
Methods: Methoxychalcones were synthesized and their structures were confirmed by NMR spectral data analyses. Evaluations of antimicrobial activity were performed against five species of Candida, two Gram-negative and five Gram-positive species. For antiproliferative activity, methoxychalcones were evaluated against four human tumorigenic cell lines, as well as human non-tumorigenic keratinocytes. Drug-likeness and pharmacokinetics properties were predicted using Molinspiration and PreADMET toolkits.
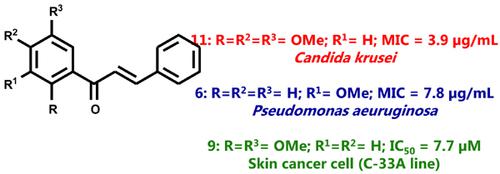
Results: In general, chalcones of series I are the most potent antifungal, antibacterial and antiproliferative agents. 3’, 4’, 5’-Trimethoxychalcone (12) demonstrated potent antifungal activity against Candida krusei (MIC = 3.9 μg/mL), eight times more potent than fluconazole (reference antifungal drug). 3’-Methoxychalcone (6) displayed anti-Pseudomonas activity (MIC = 7.8 μg/mL). 2’,5’-Dimethoxychalcone (9) displayed potent antiproliferative effect against C-33A (cervix), A-431 (skin) and MCF-7 (breast), with IC50 values ranging from 7.7 to 9.2 μM. Its potency was superior to curcumin (reference antiproliferative compound), which exhibited IC50 values ranging from 10.4 to 19.0 μM.
Conclusion: Our studies corroborated the relevance of methoxychalcones as antifungal, antibacterial and antiproliferative agents. In addition, we elucidated influence of the position and number of methoxyl groups toward bioactivity. In silico predictions indicated good drug-likeness and pharmacokinetics properties to the library of methoxychalcones.
中文翻译:

甲氧基查耳酮:甲氧基对抗真菌,抗菌和抗增殖活性的影响
背景:被甲氧基取代的查耳酮具有广泛的生物活性,包括抗真菌,抗菌和抗增殖作用。然而,尚未描述关于该取代基在查耳酮骨架上的相关性的明确和明确的研究。
目的:这项工作的目的是评估两个系列的十七种合成的区域异构甲氧基查耳酮的抑菌,抗真菌和抗增殖活性。系列I和II由分别被环A(5-12)和B(13-21)上的甲氧基取代的查耳酮构成。此外,甲氧基查耳酮的文库已提交计算机模拟药物和药代动力学性质的预测。
方法:合成甲氧基查耳酮,并通过NMR光谱数据分析确定其结构。对五种念珠菌,两种革兰氏阴性菌和五种革兰氏阳性菌进行了抗菌活性评估。对于抗增殖活性,针对四种人类致瘤细胞系以及人类非致瘤性角质形成细胞评估了甲氧基查耳酮。使用Molinspiration和PreADMET工具包可预测药物相似性和药代动力学特性。
结果:通常,系列I的查耳酮是最有效的抗真菌,抗菌和抗增殖剂。3',4',5'-三甲氧基查耳酮(12)表现出对克鲁斯假丝酵母的有效抗真菌活性(MIC = 3.9μg/ mL),比氟康唑(参考抗真菌药)的效力强八倍。3'-甲氧基查耳酮(6)显示出抗假单胞菌活性(MIC = 7.8μg/ mL)。2',5'-二甲氧基查耳酮(9)对C-33A(宫颈),A-431(皮肤)和MCF-7(乳腺)表现出有效的抗增殖作用,IC50值为7.7至9.2μM。其效力优于姜黄素(参考抗增殖化合物),后者的IC50值范围为10.4至19.0μM。
结论:我们的研究证实了甲氧基查耳酮作为抗真菌剂,抗菌剂和抗增殖剂的相关性。另外,我们阐明了甲氧基的位置和数量对生物活性的影响。在计算机上的预测表明对甲氧基查耳酮的文库具有良好的药物相似性和药代动力学性质。


























 京公网安备 11010802027423号
京公网安备 11010802027423号