Medicinal Chemistry ( IF 2.3 ) Pub Date : 2020-10-31 , DOI: 10.2174/1573406415666190724142951 Aida Iraji 1 , Mahsima Khoshneviszadeh 1 , Pegah Bakhshizadeh 1 , Najmeh Edraki 1 , Mehdi Khoshneviszadeh 1
|
Background: Melanogenesis is a process of melanin synthesis, which is a primary response for the pigmentation of human skin. Tyrosinase is a key enzyme, which catalyzes a ratelimiting step of the melanin formation. Natural products have shown potent inhibitors, but some of these possess toxicity. Numerous synthetic inhibitors have been developed in recent years may lead to the potent anti– tyrosinase agents.
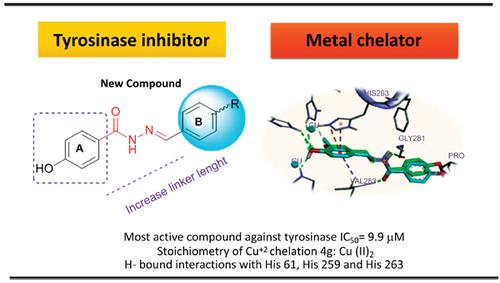
Objective: A number of 4-hydroxy-N'-methylenebenzohydrazide analogues with related structure to chalcone and tyrosine were constructed with various substituents at the benzyl ring of the molecule and evaluate as a tyrosinase inhibitor. In addition, computational analysis and metal chelating potential have been evaluated.
Methods: Design and synthesized compounds were evaluated for activity against mushroom tyrosinase. The metal chelating capacity of the potent compound was examined using the mole ratio method. Molecular docking of the synthesized compounds was carried out into the tyrosine active site.
Results: Novel 4-hydroxy-N'-methylenebenzohydrazide derivatives were synthesized. The two compounds 4c and 4g showed an IC50 near the positive control, led to a drastic inhibition of tyrosinase. Confirming in vitro results were performed via the molecular docking analysis demonstrating hydrogen bound interactions of potent compounds with histatidine-Cu+2 residues with in the active site. Kinetic study of compound 4g showed competitive inhibition towards tyrosinase. Metal chelating assay indicates the mole fraction of 1:2 stoichiometry of the 4g-Cu2+ complex.
Conclusion: The findings in the present study demonstrate that 4-Hydroxy-N'- methylenebenzohydrazide scaffold could be regarded as a bioactive core inhibitor of tyrosinase and can be used as an inspiration for further studies in this area.
中文翻译:

基于结构的设计,合成,生物学评估和分子对接研究的4-羟基-N'-亚甲基苯并肼衍生物作为酪氨酸酶抑制剂具有较强的抗黑素生成活性。
背景:黑色素生成是黑色素合成的过程,是人类皮肤色素沉着的主要反应。酪氨酸酶是关键酶,其催化黑色素形成的限速步骤。天然产物显示出有效的抑制剂,但其中一些具有毒性。近年来已经开发出许多合成抑制剂,可能导致有效的抗酪氨酸酶药物。
目的:构建了一些与查尔酮和酪氨酸相关结构的4-羟基-N'-亚甲基苯并肼类似物,该类似物在分子的苄环上带有多个取代基,并被用作酪氨酸酶抑制剂。另外,已经评估了计算分析和金属螯合潜力。
方法:评估设计和合成的化合物对蘑菇酪氨酸酶的活性。使用摩尔比方法检查有效化合物的金属螯合能力。合成化合物的分子对接在酪氨酸活性位点进行。
结果:合成了新型的4-羟基-N'-亚甲基苯并肼衍生物。两种化合物4c和4g在阳性对照附近显示出IC50,导致酪氨酸酶的强烈抑制。通过分子对接分析证实了体外结果,证明了有效化合物与带有活性位点的Histatidine-Cu + 2残基的氢键相互作用。化合物4g的动力学研究表明对酪氨酸酶具有竞争性抑制作用。金属螯合测定表明4g-Cu2 +配合物的化学计量比为1:2的摩尔分数。
结论:本研究结果表明4-羟基-N'-亚甲基苯并肼基支架可被视为酪氨酸酶的生物活性核心抑制剂,可为该领域的进一步研究提供灵感。


























 京公网安备 11010802027423号
京公网安备 11010802027423号