当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Comparative analysis of ethanol dynamics in aqueous and non-aqueous solutions
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2020-09-24 , DOI: 10.1039/d0cp03160g Ivo Jukić 1, 2, 3, 4, 5 , Martina Požar 1, 2, 3, 4, 5 , Bernarda Lovrinčević 1, 2, 3, 4, 5
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2020-09-24 , DOI: 10.1039/d0cp03160g Ivo Jukić 1, 2, 3, 4, 5 , Martina Požar 1, 2, 3, 4, 5 , Bernarda Lovrinčević 1, 2, 3, 4, 5
Affiliation
|
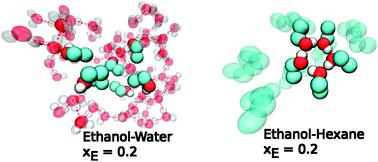
In this study, we compare the results for vibrational, reorientational and hydrogen bond dynamics of ethanol in water and in hexane across the whole concentration range. Water and hexane are both commonly used as solvents, but so far, it has been unclear to what extent they modify the solute dynamics. Ethanol is chosen as the solute because it is an aliphatic molecule that is miscibile with both solvents. It is known that ethanol forms micelle-like domains in water and cyclic clusters resembling loops in hexane. This structural micro-heterogeneity is well known both in experiments and in simulations. The main question we raise here is: is there a signature of micro-heterogeneity in the dynamical quantities of ethanol? We focus on quantities such as the vibrational spectra, the reorientational correlation functions, the self-diffusion coefficients, the ethanol–ethanol hydrogen bond correlation functions and the corresponding hydrogen bond histograms. For the first time ever, we compute the van Hove functions to reveal the dynamical variations of spatial correlations in these systems. All these results complement each other and provide a unifying dynamical description of ethanol in binary mixtures.
中文翻译:

水溶液和非水溶液中乙醇动力学的比较分析
在这项研究中,我们比较了在整个浓度范围内,乙醇在水和己烷中的振动,重整和氢键动力学的结果。水和己烷都是常用的溶剂,但到目前为止,尚不清楚它们在多大程度上改变了溶质动力学。选择乙醇作为溶质是因为它是一种脂族分子,可与两种溶剂混溶。众所周知,乙醇会在水中形成类似胶束的结构域,并形成类似于己烷中环的环状簇。这种结构的微异质性在实验和模拟中都是众所周知的。我们在这里提出的主要问题是:乙醇的动态量中是否存在微异质性的特征?我们专注于振动光谱,方向相关函数,自扩散系数,乙醇-乙醇氢键的相关函数和相应的氢键直方图。我们首次计算了van Hove函数,以揭示这些系统中空间相关性的动态变化。所有这些结果相互补充,并提供了二元混合物中乙醇的统一动力学描述。
更新日期:2020-10-19
中文翻译:

水溶液和非水溶液中乙醇动力学的比较分析
在这项研究中,我们比较了在整个浓度范围内,乙醇在水和己烷中的振动,重整和氢键动力学的结果。水和己烷都是常用的溶剂,但到目前为止,尚不清楚它们在多大程度上改变了溶质动力学。选择乙醇作为溶质是因为它是一种脂族分子,可与两种溶剂混溶。众所周知,乙醇会在水中形成类似胶束的结构域,并形成类似于己烷中环的环状簇。这种结构的微异质性在实验和模拟中都是众所周知的。我们在这里提出的主要问题是:乙醇的动态量中是否存在微异质性的特征?我们专注于振动光谱,方向相关函数,自扩散系数,乙醇-乙醇氢键的相关函数和相应的氢键直方图。我们首次计算了van Hove函数,以揭示这些系统中空间相关性的动态变化。所有这些结果相互补充,并提供了二元混合物中乙醇的统一动力学描述。



























 京公网安备 11010802027423号
京公网安备 11010802027423号