当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Evidence for a stereoselective mechanism for bitopic activity by extended-length antagonists of the D3 dopamine receptor.
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2020-09-24 , DOI: 10.1021/acschemneuro.0c00425 Amy E Moritz 1 , Alessandro Bonifazi 2 , Adrian M Guerrero 2 , Vivek Kumar 2 , R Benjamin Free 1 , J Robert Lane 3 , Ravi Kumar Verma 4 , Lei Shi 4 , Amy Hauck Newman 2 , David R Sibley 1
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2020-09-24 , DOI: 10.1021/acschemneuro.0c00425 Amy E Moritz 1 , Alessandro Bonifazi 2 , Adrian M Guerrero 2 , Vivek Kumar 2 , R Benjamin Free 1 , J Robert Lane 3 , Ravi Kumar Verma 4 , Lei Shi 4 , Amy Hauck Newman 2 , David R Sibley 1
Affiliation
|
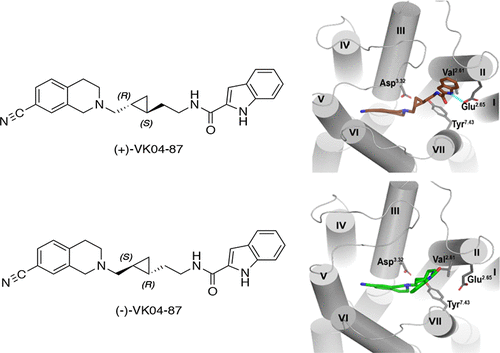
The D3 dopamine receptor (D3R) has been suggested as a drug target for the treatment of a number of neuropsychiatric disorders, including substance use disorders (SUD). Many D3R-selective antagonists are bivalent in nature in that they engage two distinct sites on the receptor—a primary pharmacophore binds to the orthosteric site, where dopamine binds, whereas a secondary pharmacophore interacts with a unique secondary binding pocket (SBP). When engagement of the secondary pocket exerts allosteric activity, the compound is said to be bitopic. We recently reported the synthesis and characterization of two bitopic antagonists of the D3R, (±)-VK04-87 and (±)-VK05-95, which incorporated a racemic trans-cyclopropylmethyl linking chain. To gain a better understanding of the role of chirality in determining the pharmacology of such compounds, we resolved the enantiomers of (±)-VK04-87. We found that the (+)-isomer displays higher affinity for the D3R and exhibits greater selectivity versus the D2R than the (−)-isomer. Strikingly, using functional assays, we found that (+)-VK04-87 inhibits the D3R in a noncompetitive manner, while (−)-VK04-87 behaves as a purely competitive antagonist, indicating that the apparent allosteric activity of the racemate is due to the (+)-isomer. Molecular dynamic simulations of (+)-VK04-87 and (−)-VK04-87 binding to the D3R suggest that the (+)-isomer is able to interact with the SBP of the receptor whereas the (−)-isomer bends away from this pocket, thus potentially explaining their differing pharmacology. These results emphasize the importance of the linker, and its isomeric conformations, within extended-length molecules for their positioning and engagement within GPCR binding pockets.
中文翻译:

D3 多巴胺受体的长效拮抗剂对双位活性的立体选择性机制的证据。
D3 多巴胺受体 (D3R) 已被建议作为治疗多种神经精神疾病的药物靶点,包括物质使用障碍 (SUD)。许多 D3R 选择性拮抗剂本质上是二价的,因为它们与受体上的两个不同位点结合——主要药效团与多巴胺结合的正构位点结合,而次要药效团与独特的二级结合口袋 (SBP) 相互作用。当第二口袋的接合发挥变构活性时,该化合物被称为双位的。我们最近报道了 D3R 的两种双位拮抗剂 (±)-VK04-87 和 (±)-VK05-95 的合成和表征,它们结合了外消旋反式-环丙基甲基连接链。为了更好地了解手性在确定此类化合物的药理学方面的作用,我们解析了 (±)-VK04-87 的对映异构体。我们发现 (+)-异构体对 D3R 显示出更高的亲和力,并且与 (-)-异构体相比,对 D2R 表现出更高的选择性。引人注目的是,使用功能分析,我们发现 (+)-VK04-87 以非竞争性方式抑制 D3R,而 (-)-VK04-87 表现为纯粹的竞争性拮抗剂,表明外消旋体的明显变构活性是由于(+)-异构体。(+)-VK04-87 和 (-)-VK04-87 与 D3R 结合的分子动力学模拟表明 (+)-异构体能够与受体的 SBP 相互作用,而 (-)-异构体会弯曲从这个口袋里,从而可能解释他们不同的药理学。
更新日期:2020-10-21
中文翻译:

D3 多巴胺受体的长效拮抗剂对双位活性的立体选择性机制的证据。
D3 多巴胺受体 (D3R) 已被建议作为治疗多种神经精神疾病的药物靶点,包括物质使用障碍 (SUD)。许多 D3R 选择性拮抗剂本质上是二价的,因为它们与受体上的两个不同位点结合——主要药效团与多巴胺结合的正构位点结合,而次要药效团与独特的二级结合口袋 (SBP) 相互作用。当第二口袋的接合发挥变构活性时,该化合物被称为双位的。我们最近报道了 D3R 的两种双位拮抗剂 (±)-VK04-87 和 (±)-VK05-95 的合成和表征,它们结合了外消旋反式-环丙基甲基连接链。为了更好地了解手性在确定此类化合物的药理学方面的作用,我们解析了 (±)-VK04-87 的对映异构体。我们发现 (+)-异构体对 D3R 显示出更高的亲和力,并且与 (-)-异构体相比,对 D2R 表现出更高的选择性。引人注目的是,使用功能分析,我们发现 (+)-VK04-87 以非竞争性方式抑制 D3R,而 (-)-VK04-87 表现为纯粹的竞争性拮抗剂,表明外消旋体的明显变构活性是由于(+)-异构体。(+)-VK04-87 和 (-)-VK04-87 与 D3R 结合的分子动力学模拟表明 (+)-异构体能够与受体的 SBP 相互作用,而 (-)-异构体会弯曲从这个口袋里,从而可能解释他们不同的药理学。



























 京公网安备 11010802027423号
京公网安备 11010802027423号