当前位置:
X-MOL 学术
›
Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of C-terminal glycine-rich o-aminoanilide peptides without overacylation for use in benzotriazole-mediated native chemical ligation
Peptide Science ( IF 2.4 ) Pub Date : 2020-09-23 , DOI: 10.1002/pep2.24194 Fernando J. Ferrer‐Gago 1 , Li Quan Koh 1
Peptide Science ( IF 2.4 ) Pub Date : 2020-09-23 , DOI: 10.1002/pep2.24194 Fernando J. Ferrer‐Gago 1 , Li Quan Koh 1
Affiliation
|
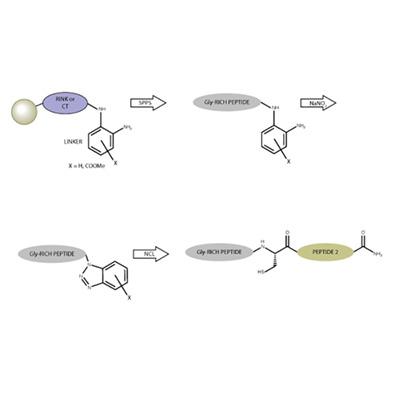
Native chemical ligation (NCL) requires the constant development of methods that facilitate the efficient synthesis of C-terminal peptide thioesters, which are key intermediates in NCL. After testing several resins and linkers, we have developed a solid support on the Rink and chlorotrityl chloride resins based on the attachment of o-phenylenediamine (PheDA), with and without an electron withdrawing group. These linkers enable the synthesis of C-terminus o-aminoanilide peptides without overacylation when the glycine is the first amino acid in the synthesis or when we have a glycine-rich sequence. Upon treatment with NaNO2, the glycine o-aminoanilide peptides produce C-terminus benzotriazole (Bt) peptides. The C-terminus-Bt peptides undergo thiol exchange, yielding thioester peptides, which can then be used in an NCL reaction. The linkers attached to the Rink and CT resins efficiently avoid overacylation in the synthesis of glycine-rich sequences, increasing the yield and purity of these sequences.
中文翻译:

无需过度酰化即可合成C端富含甘氨酸的邻氨基苯胺肽,用于苯并三唑介导的天然化学连接
天然化学连接(NCL)需要不断开发有助于有效合成C端肽硫酯的方法,而C端肽硫酯是NCL的关键中间体。在测试了几种树脂和连接基之后,我们基于邻苯二胺(PheDA)的连接,在有和没有吸电子基团的情况下,在Rink和氯三苯甲基氯树脂上开发了一种固相支持物。当甘氨酸是合成中的第一个氨基酸或当我们具有富含甘氨酸的序列时,这些接头能够合成C端邻氨基苯胺肽而不会过度酰化。当与治疗的NaNO 2,甘氨酸Ò-氨基苯胺肽产生C端苯并三唑(Bt)肽。C末端Bt肽进行巯基交换,生成硫酯肽,然后可将其用于NCL反应中。连接到Rink和CT树脂上的接头有效地避免了富含甘氨酸序列合成中的过度酰化,从而提高了这些序列的收率和纯度。
更新日期:2020-09-23
中文翻译:

无需过度酰化即可合成C端富含甘氨酸的邻氨基苯胺肽,用于苯并三唑介导的天然化学连接
天然化学连接(NCL)需要不断开发有助于有效合成C端肽硫酯的方法,而C端肽硫酯是NCL的关键中间体。在测试了几种树脂和连接基之后,我们基于邻苯二胺(PheDA)的连接,在有和没有吸电子基团的情况下,在Rink和氯三苯甲基氯树脂上开发了一种固相支持物。当甘氨酸是合成中的第一个氨基酸或当我们具有富含甘氨酸的序列时,这些接头能够合成C端邻氨基苯胺肽而不会过度酰化。当与治疗的NaNO 2,甘氨酸Ò-氨基苯胺肽产生C端苯并三唑(Bt)肽。C末端Bt肽进行巯基交换,生成硫酯肽,然后可将其用于NCL反应中。连接到Rink和CT树脂上的接头有效地避免了富含甘氨酸序列合成中的过度酰化,从而提高了这些序列的收率和纯度。



























 京公网安备 11010802027423号
京公网安备 11010802027423号