当前位置:
X-MOL 学术
›
Acta Cryst. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Triple molybdates K3–xNa1+xM4(MoO4)6 (M = Ni, Mg, Co) and K3+xLi1–xMg4(MoO4)6 isotypic with II‐Na3Fe2(AsO4)3 and yurmarinite: synthesis, potassium disorder, crystal chemistry and ionic conductivity
Acta Crystallographica Section B ( IF 2.684 ) Pub Date : 2020-09-23 , DOI: 10.1107/s2052520620010677 Oksana A. Gulyaeva , Zoya A. Solodovnikova , Sergey F. Solodovnikov , Evgeniya S. Zolotova , Yuliya G. Mateyshina , Nikolai F. Uvarov
Acta Crystallographica Section B ( IF 2.684 ) Pub Date : 2020-09-23 , DOI: 10.1107/s2052520620010677 Oksana A. Gulyaeva , Zoya A. Solodovnikova , Sergey F. Solodovnikov , Evgeniya S. Zolotova , Yuliya G. Mateyshina , Nikolai F. Uvarov
|
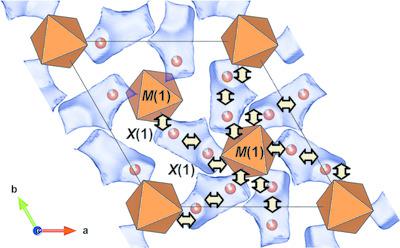
The triple molybdates K3–xNa1+xM4(MoO4)6 (M = Ni, Mg, Co) and K3+xLi1–xMg4(MoO4)6 were found upon studying the corresponding ternary molybdate systems, and their structures, thermal stability and electrical conductiviplusmnty were investigated. The compounds crystallize in the space group R3c and are isostructural with the sodium‐ion conductor II‐Na3Fe2(AsO4)3 and yurmarinite, Na7(Fe3+, Mg, Cu)4(AsO4)6; their basic structural units are flat polyhedral clusters of the central M1O6 octahedron sharing edges with three surrounding M2O6 octahedra, which combine with single NaO6 octahedra and bridging MoO4 tetrahedra to form open three‐dimensional (3D) frameworks where the cavities are partially occupied by disordered potassium (sodium) ions. The split alkali‐ion positions in K3–xNa1+xM4(MoO4)6 (M = Ni, Mg, Co) give their structural formulae as [(K,Na)0.5□0.5)]6(Na)[M1][M2]3(MoO4)6, whereas the lithium‐containing compound (K0.5□0.5)6(Mg0.89K0.11)(Li0.89Mg0.11)Mg3(MoO4)6 shows an unexpected (Mg, K) isomorphism, which is similar to (Mn, K) and (Co, K) substitutions in isostructural K3+xLi1–xM4(MoO4)6 (M = Mn, Co). The crystal chemistry of the title compounds and related arsenates, phosphates and molybdates was considered, and the connections of the cationic distributions with potential 3D ionic conductivity were shown by means of calculating the bond valence sum (BVS) maps for the Na+, Li+ and K+ ions. Electrical conductivity measurements gave relatively low values for the triple molybdates [σ = 4.8 × 10−6 S cm−1 at 390°C for K3NaCo4(MoO4)6 and 5 × 10−7 S cm−1 at 400°C for K3LiMg4(MoO4)6] compared with II‐Na3Fe2(AsO4)3 (σ = 8.3 × 10−4 S cm−1 at 300°C). This may be explained by a low concentration of sodium or lithium ions and the blocking of their transport by large potassium ions.
中文翻译:

同型同型II-Na3Fe2(AsO4)3和褐铁矿的三钼酸盐K3-xNa1 + xM4(MoO4)6(M = Ni,Mg,Co)和K3 + xLi1-xMg4(MoO4)6同型:合成,钾无序,晶体化学和离子电导率
通过研究相应的三元化合物,发现了三钼酸盐K 3– x Na 1+ x M 4(MoO 4)6(M = Ni,Mg,Co)和K 3+ x Li 1– x Mg 4(MoO 4)6。研究了钼酸盐体系及其结构,热稳定性和导电性。这些化合物在空间群R 3 c中结晶,并与钠离子导体II-Na 3 Fe 2(AsO 4)3同构褐铁矿,Na 7(Fe 3+,Mg,Cu)4(AsO 4)6;它们的基本结构单元是中央M 1O 6八面体的平坦多面体簇,与三个周围的M 2O 6八面体共享边,并与单个NaO 6八面体结合并桥接MoO 4四面体以形成开放的三维(3D)框架,其中的空腔部分被无序的钾(钠)离子占据。在K 3– x Na 1+ x M 4(MoO 4)6(M = Ni,Mg,Co)的结构式为[(K,Na)0.5 □ 0.5)] 6(Na)[ M 1] [ M 2] 3(MoO 4)6,而锂含有化合物(K 0.5 □ 0.5)6(Mg 0.89 K 0.11)(Li 0.89 Mg 0.11)Mg 3(MoO 4)6表现出意外的(Mg,K)同构,类似于(Mn,K)和(Co ,K)等结构K 3+ x中的取代Li 1– x M 4(MoO 4)6(M = Mn,Co)。考虑了标题化合物及其相关的砷酸盐,磷酸盐和钼酸盐的晶体化学性质,并通过计算Na +,Li +的键合价和(BVS)图,显示了阳离子分布与潜在3D离子电导率的联系。和K +离子 对于K 3 NaCo 4(MoO 4)6,在390°C下,三价钼酸盐的电导率测量值相对较低[σ= 4.8×10 -6 S cm -1K 3 LiMg 4(MoO 4)6 ]和II-Na 3 Fe 2(AsO 4)3在400°C时为5×10 -7 S cm -1(σ= 8.3×10 -4 S cm -1在300°C下)。钠或锂离子浓度低以及大钾离子阻碍了它们的运输,可以解释这一点。
更新日期:2020-10-07
中文翻译:

同型同型II-Na3Fe2(AsO4)3和褐铁矿的三钼酸盐K3-xNa1 + xM4(MoO4)6(M = Ni,Mg,Co)和K3 + xLi1-xMg4(MoO4)6同型:合成,钾无序,晶体化学和离子电导率
通过研究相应的三元化合物,发现了三钼酸盐K 3– x Na 1+ x M 4(MoO 4)6(M = Ni,Mg,Co)和K 3+ x Li 1– x Mg 4(MoO 4)6。研究了钼酸盐体系及其结构,热稳定性和导电性。这些化合物在空间群R 3 c中结晶,并与钠离子导体II-Na 3 Fe 2(AsO 4)3同构褐铁矿,Na 7(Fe 3+,Mg,Cu)4(AsO 4)6;它们的基本结构单元是中央M 1O 6八面体的平坦多面体簇,与三个周围的M 2O 6八面体共享边,并与单个NaO 6八面体结合并桥接MoO 4四面体以形成开放的三维(3D)框架,其中的空腔部分被无序的钾(钠)离子占据。在K 3– x Na 1+ x M 4(MoO 4)6(M = Ni,Mg,Co)的结构式为[(K,Na)0.5 □ 0.5)] 6(Na)[ M 1] [ M 2] 3(MoO 4)6,而锂含有化合物(K 0.5 □ 0.5)6(Mg 0.89 K 0.11)(Li 0.89 Mg 0.11)Mg 3(MoO 4)6表现出意外的(Mg,K)同构,类似于(Mn,K)和(Co ,K)等结构K 3+ x中的取代Li 1– x M 4(MoO 4)6(M = Mn,Co)。考虑了标题化合物及其相关的砷酸盐,磷酸盐和钼酸盐的晶体化学性质,并通过计算Na +,Li +的键合价和(BVS)图,显示了阳离子分布与潜在3D离子电导率的联系。和K +离子 对于K 3 NaCo 4(MoO 4)6,在390°C下,三价钼酸盐的电导率测量值相对较低[σ= 4.8×10 -6 S cm -1K 3 LiMg 4(MoO 4)6 ]和II-Na 3 Fe 2(AsO 4)3在400°C时为5×10 -7 S cm -1(σ= 8.3×10 -4 S cm -1在300°C下)。钠或锂离子浓度低以及大钾离子阻碍了它们的运输,可以解释这一点。


























 京公网安备 11010802027423号
京公网安备 11010802027423号