当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rh‐Catalyzed Decarbonylative Cross‐Coupling between o‐Carboranes and Twisted Amides: A Regioselective, Additive‐Free, and Concise Late‐Stage Carboranylation
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2020-09-23 , DOI: 10.1002/chem.202003634 Chun-Xiao Li 1 , Qian Ning 1 , Wenxuan Zhao 1 , Hou-Ji Cao 1 , Yi-Ping Wang 1 , Hong Yan 1 , Chang-Sheng Lu 1 , Yong Liang 1
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2020-09-23 , DOI: 10.1002/chem.202003634 Chun-Xiao Li 1 , Qian Ning 1 , Wenxuan Zhao 1 , Hou-Ji Cao 1 , Yi-Ping Wang 1 , Hong Yan 1 , Chang-Sheng Lu 1 , Yong Liang 1
Affiliation
|
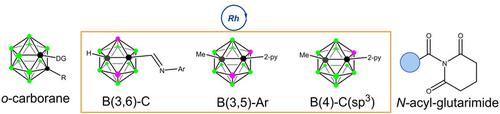
The convenient cross‐coupling of sp2 or sp3 carbons with a specific boron vertex on carborane cage represents significant synthetic values and insurmountable challenges. In this work, we report an Rh‐catalyzed reaction between o‐carborane and N‐acyl‐glutarimides to construct various Bcage−C bonds. Under the optimized condition, the removable imine directing group (DG) leads to B(3)− or B(3,6)−C couplings, while the pyridyl DG leads to B(3,5)−Ar coupling. In particular, an unexpected rearrangement of amide reagent is observed in pyridyl directed B(4)−C(sp3) formation. This scalable protocol has many advantages, including easy access, the use of cheap and widely available coupling agents, no requirement of an external ligand, base or oxidant, high efficiency, and a broad substrate scope. Leveraging the RhI dimer and twisted amides, this method enables straightforward access to diversely substituted and therapeutically important carborane derivatives at boron site, and provides a highly valuable vista for carborane‐based drug screening.
中文翻译:

邻位碳硼烷和扭曲酰胺之间的Rh催化的脱羰交叉偶联:区域选择性,无添加剂且简洁的晚期碳硼烷基化
sp 2或sp 3碳与碳硼烷笼上的特定硼顶点的便捷交叉偶联代表了重要的合成价值和不可克服的挑战。在这项工作中,我们报道了邻-卡甲烷与N-酰基-戊二酰亚胺之间的Rh催化反应,以构建各种B笼-C键。在优化的条件下,可移动的亚胺导向基团(DG)导致B(3)-或B(3,6)-C偶联,而吡啶基DG导致B(3,5)-Ar偶联。特别是在吡啶基定向的B(4)-C(sp 3)中观察到酰胺试剂的意外重排)形成。这种可扩展的协议具有许多优势,包括易于访问,使用便宜且可广泛使用的偶联剂,不需要外部配体,碱或氧化剂,高效,广泛的底物范围。利用Rh I二聚体和扭曲的酰胺,该方法可以直接在硼位点获得各种取代的且对治疗具有重要意义的碳硼烷衍生物,并为基于碳硼烷的药物筛选提供了极有价值的前景。
更新日期:2020-09-23
中文翻译:

邻位碳硼烷和扭曲酰胺之间的Rh催化的脱羰交叉偶联:区域选择性,无添加剂且简洁的晚期碳硼烷基化
sp 2或sp 3碳与碳硼烷笼上的特定硼顶点的便捷交叉偶联代表了重要的合成价值和不可克服的挑战。在这项工作中,我们报道了邻-卡甲烷与N-酰基-戊二酰亚胺之间的Rh催化反应,以构建各种B笼-C键。在优化的条件下,可移动的亚胺导向基团(DG)导致B(3)-或B(3,6)-C偶联,而吡啶基DG导致B(3,5)-Ar偶联。特别是在吡啶基定向的B(4)-C(sp 3)中观察到酰胺试剂的意外重排)形成。这种可扩展的协议具有许多优势,包括易于访问,使用便宜且可广泛使用的偶联剂,不需要外部配体,碱或氧化剂,高效,广泛的底物范围。利用Rh I二聚体和扭曲的酰胺,该方法可以直接在硼位点获得各种取代的且对治疗具有重要意义的碳硼烷衍生物,并为基于碳硼烷的药物筛选提供了极有价值的前景。



























 京公网安备 11010802027423号
京公网安备 11010802027423号