Journal of Structural Biology ( IF 3 ) Pub Date : 2020-09-24 , DOI: 10.1016/j.jsb.2020.107632 So-Hee Jin 1 , Haehee Lee 1 , Yongho Shin 1 , Jeong-Han Kim 1 , Sangkee Rhee 2
|
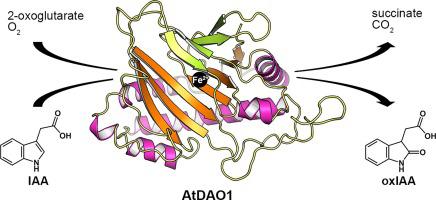
Indole-3-acetic acid (IAA), the major form of the plant hormone auxin, regulates almost every aspect of plant growth and development. Therefore, auxin homeostasis is an essential process in plants. Different metabolic routes are involved in auxin homeostasis, but the catabolic pathway has remained elusive until recent studies identified DIOXYGENASE FOR AUXIN OXIDATION (DAO) from rice and Arabidopsis thaliana. DAO, a member of the 2-oxoglutarate/Fe(II)-dependent oxygenase (2ODO) family, constitutes a major enzyme for IAA catabolism. This enzyme catalyzes, with the cosubstrate 2-oxoglutarate, the conversion of IAA into 2-oxoindole-3-acetic acid, a functionally inactive oxidative product of IAA. Here, we report a crystal structure of the unliganded DAO1 from A. thaliana (AtDAO1) and its complex with 2-oxoglutarate. AtDAO1 is structurally homologous with members of the 2ODO family but exhibits unique features in the prime substrate IAA binding site. We provide structural analyses of a putative binding site for IAA, supporting possible structural determinants for the substrate specificity of AtDAO1 toward IAA.
中文翻译:

拟南芥中吲哚-3-乙酸分解代谢酶 DAO1 的晶体结构
吲哚-3-乙酸 (IAA) 是植物激素生长素的主要形式,几乎调节植物生长和发育的各个方面。因此,生长素稳态是植物中必不可少的过程。不同的代谢途径参与植物生长素稳态,但分解代谢途径一直难以捉摸,直到最近的研究从水稻和拟南芥中鉴定出生长素氧化的双氧酶( DAO ) 。DAO 是 2-酮戊二酸/Fe(II) 依赖性加氧酶 (2ODO) 家族的成员,是 IAA 分解代谢的主要酶。该酶与共底物 2-酮戊二酸一起催化 IAA 转化为 2-oxoindole-3-乙酸,IAA 的一种功能失活的氧化产物。在这里,我们报告了未配体 DAO1 的晶体结构A. thaliana (AtDAO1) 及其与 2-酮戊二酸的复合物。AtDAO1 在结构上与 2ODO 家族的成员同源,但在主要底物 IAA 结合位点中表现出独特的特征。我们提供了 IAA 假定结合位点的结构分析,支持 AtDAO1 对 IAA 的底物特异性的可能结构决定因素。



























 京公网安备 11010802027423号
京公网安备 11010802027423号