当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An Improved Process for the Manufacture of 5′-O-(4,4′-Dimethoxytrityl)-N2-isobutyryl-2′-O-(2-methoxyethyl)guanosine
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2020-09-23 , DOI: 10.1021/acs.oprd.0c00261 Andrew K. McPherson 1 , Daniel Capaldi 1 , Lijian Chen 1 , Philip Olsen 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2020-09-23 , DOI: 10.1021/acs.oprd.0c00261 Andrew K. McPherson 1 , Daniel Capaldi 1 , Lijian Chen 1 , Philip Olsen 1
Affiliation
|
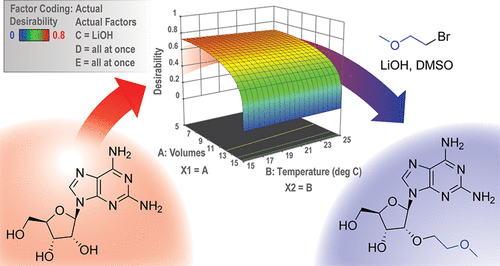
A revised, optimized process for the manufacture of 5′-O-(4,4′-dimethoxytrityl)-N2-isobutyryl-2′-O-(2-methoxyethyl)guanosine (MOE G PNS) that controls critical impurities to less than 0.2% was developed. The 2′-O-alkylation of 2,6-diaminopurine riboside (DAPR) with 1-bromo-2-methoxyethane (MOE-Br) in DMSO was examined using a design of experiments approach, which led to the selection of LiOH as the optimal base choice. Equivalents of base and MOE-Br were optimized to control levels of the critical 3′-O-(2-methoxyethyl) impurity and residual DAPR. DMSO was removed with a solid-phase extraction using SP-207 resin, affording the desired 2′-O-(2-methoxyethyl)-2,6-diaminopurine riboside (MOE DAPR) in an aqueous solution in 53% yield. The methyl bromide impurity commonly found in bulk sourced MOE-Br was controlled using a codistillation with MEK, thereby controlling the resulting critical 2′-O-methyl nucleoside impurity. The aqueous solution was telescoped into the enzymatic conversion of MOE DAPR to 2′-O-(2-methoxyethyl)guanosine (MOE G) using adenosine deaminase. MOE G purity is enhanced at this step due to enzymatic selectivity and crystallization from the reaction mixture, which together significantly reduce levels of the 3′ MOE G isomer. Installation of the isobutyryl and 4,4-dimethoxytrityl protecting groups proceeded similarly to published literature methods, but the workup and isolation conditions were optimized. A series of extractions after isobutyrlation were used to control critical and noncritical impurities, and the final product was crystallized to upgrade purity. The improved process was carried out at the production scale to afford 59 kg of MOE G PNS with no critical impurity over 0.2% in an overall yield of 26%.
中文翻译:

5'- O-(4,4'-二甲氧基三苯甲基)-N 2-异丁酰基-2'- O-(2-甲氧基乙基)鸟苷的制备方法的改进
经过修订的优化工艺,用于生产5'- O-(4,4'-二甲氧基三苯甲基)-N 2-异丁酰基-2'- O-(2-甲氧基乙基)鸟苷(MOE G PNS),可将杂质降至最低超过了0.2%。使用设计的实验方法检查了DMSO中2,6-二氨基嘌呤核糖苷(DAPR)与1-溴-2-甲氧基乙烷(MOE-Br)的2' - O-烷基化反应,从而选择了LiOH作为最佳基础选择。优化了碱和MOE-Br的当量,以控制关键的3' - O-(2-甲氧基乙基)杂质和残留DAPR的水平。使用SP-207树脂通过固相萃取去除DMSO,得到所需的2'- O-(2-甲氧基乙基)-2,6-二氨基嘌呤核糖苷(MOE DAPR)在水溶液中的产率为53%。通过与MEK共蒸馏,可控制在批量生产的MOE-Br中常见的甲基溴杂质,从而控制所得的关键2'- O-甲基核苷杂质。将该水溶液伸缩式转化为MOE DAPR到2'- O的酶促转化-(2-甲氧基乙基)鸟苷(MOE G),使用腺苷脱氨酶。由于该反应混合物的酶促选择性和结晶作用,MOE G纯度在此步骤得到提高,这一起显着降低了3'MOE G异构体的含量。异丁酰基和4,4-二甲氧基三苯甲基保护基的安装方法与已公开的文献方法相似,但是对后处理和分离条件进行了优化。异丁酰化后的一系列萃取用于控制关键和非关键杂质,使最终产物结晶以提高纯度。改进的方法在生产规模上进行,得到59 kg的MOE G PNS,其关键杂质含量不超过0.2%,总收率为26%。
更新日期:2020-11-21
中文翻译:

5'- O-(4,4'-二甲氧基三苯甲基)-N 2-异丁酰基-2'- O-(2-甲氧基乙基)鸟苷的制备方法的改进
经过修订的优化工艺,用于生产5'- O-(4,4'-二甲氧基三苯甲基)-N 2-异丁酰基-2'- O-(2-甲氧基乙基)鸟苷(MOE G PNS),可将杂质降至最低超过了0.2%。使用设计的实验方法检查了DMSO中2,6-二氨基嘌呤核糖苷(DAPR)与1-溴-2-甲氧基乙烷(MOE-Br)的2' - O-烷基化反应,从而选择了LiOH作为最佳基础选择。优化了碱和MOE-Br的当量,以控制关键的3' - O-(2-甲氧基乙基)杂质和残留DAPR的水平。使用SP-207树脂通过固相萃取去除DMSO,得到所需的2'- O-(2-甲氧基乙基)-2,6-二氨基嘌呤核糖苷(MOE DAPR)在水溶液中的产率为53%。通过与MEK共蒸馏,可控制在批量生产的MOE-Br中常见的甲基溴杂质,从而控制所得的关键2'- O-甲基核苷杂质。将该水溶液伸缩式转化为MOE DAPR到2'- O的酶促转化-(2-甲氧基乙基)鸟苷(MOE G),使用腺苷脱氨酶。由于该反应混合物的酶促选择性和结晶作用,MOE G纯度在此步骤得到提高,这一起显着降低了3'MOE G异构体的含量。异丁酰基和4,4-二甲氧基三苯甲基保护基的安装方法与已公开的文献方法相似,但是对后处理和分离条件进行了优化。异丁酰化后的一系列萃取用于控制关键和非关键杂质,使最终产物结晶以提高纯度。改进的方法在生产规模上进行,得到59 kg的MOE G PNS,其关键杂质含量不超过0.2%,总收率为26%。



























 京公网安备 11010802027423号
京公网安备 11010802027423号