当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mn2+ Complexes Containing Sulfonamide Groups with pH-Responsive Relaxivity.
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2020-09-23 , DOI: 10.1021/acs.inorgchem.0c02098 Rocío Uzal-Varela 1 , Aurora Rodríguez-Rodríguez 1 , Miguel Martínez-Calvo 1 , Fabio Carniato 2 , Daniela Lalli 2 , David Esteban-Gómez 1 , Isabel Brandariz 1 , Paulo Pérez-Lourido 3 , Mauro Botta 2 , Carlos Platas-Iglesias 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2020-09-23 , DOI: 10.1021/acs.inorgchem.0c02098 Rocío Uzal-Varela 1 , Aurora Rodríguez-Rodríguez 1 , Miguel Martínez-Calvo 1 , Fabio Carniato 2 , Daniela Lalli 2 , David Esteban-Gómez 1 , Isabel Brandariz 1 , Paulo Pérez-Lourido 3 , Mauro Botta 2 , Carlos Platas-Iglesias 1
Affiliation
|
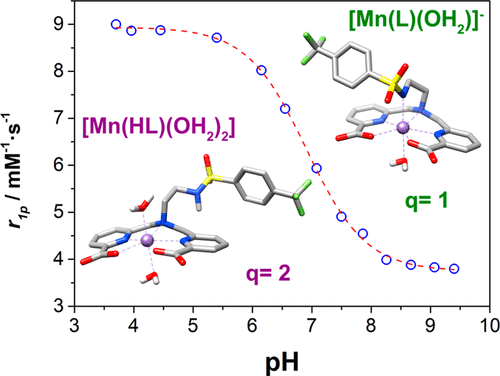
We present two ligands containing a N-ethyl-4-(trifluoromethyl)benzenesulfonamide group attached to either a 6,6′-(azanediylbis(methylene))dipicolinic acid unit (H3DPASAm) or a 2,2′-(1,4,7-triazonane-1,4-diyl)diacetic acid macrocyclic platform (H3NO2ASAm). These ligands were designed to provide a pH-dependent relaxivity response upon complexation with Mn2+ in aqueous solution. The protonation constants of the ligands and the stability constants of the Mn2+ complexes were determined using potentiometric titrations complemented by spectrophotometric experiments. The deprotonations of the sulfonamide groups of the ligands are characterized by protonation constants of log KiH = 10.36 and 10.59 for DPASAm3– and HNO2ASAm2–, respectively. These values decrease dramatically to log KiH = 6.43 and 5.42 in the presence of Mn2+, because of the coordination of the negatively charged sulfonamide groups to the metal ion. The higher log KiH value in [Mn(DPASAm)]− is related to the formation of a seven-coordinate complex, while the metal ion in [Mn(NO2ASAm)]− is six-coordinated. The X-ray crystal structure of Na[Mn(DPASAm)(H2O)]·2H2O confirms the formation of a seven-coordinate complex, where the coordination environment is fulfilled by the donor atoms of the two picolinate groups, the amine N atom, the N atom of the sulfonamide group, and a coordinated water molecule. The lower conditional stability of the [Mn(NO2ASAm)]− complex and the lower protonation constant of the sulfonamide group results in complex dissociation at relatively high pH (<7.0). However, protonation of the sulfonamide group in [Mn(DPASAm)]− falls into the physiologically relevant pH window and causes a significant increase in relaxivity from r1p = 3.8 mM–1 s–1 at pH 9.0 to r1p = 8.9 mM–1 s–1 at pH 4.0 (10 MHz, 25 °C).
中文翻译:

含磺酰胺基的Mn2 +配合物,具有pH响应弛豫性。
我们含有本两个配体Ñ附接到乙基-4-(三氟甲基)苯磺酰胺基团或者是6,6' - (azanediylbis(亚甲基))吡啶二羧酸单元(H 3 DPASAm)或2,2' - (1, 4,7-三氮烷-1,4-二基)二乙酸大环平台(H 3 NO2ASAm)。这些配体经设计可在水溶液中与Mn 2+络合后提供pH依赖性弛豫响应。配体的质子化常数和Mn 2+络合物的稳定性常数是通过分光光度法补充的电位滴定法测定的。配体的磺酰胺基团的去质子化的特征在于log K i的质子化常数对于DPASAm 3–和HNO2ASAm 2–,H分别为10.36和10.59 。由于带负电的磺酰胺基团与金属离子的配位,在Mn 2+存在下,这些值急剧下降至log K i H = 6.43和5.42 。[Mn(DPASAm)] -中较高的log K i H值与七配位络合物的形成有关,而[Mn(NO2ASAm)] -中金属离子为六配位。Na [Mn(DPASAm)(H 2 O)] · 2H 2的X射线晶体结构O证实形成七配位配合物,其中两个吡啶甲酸基团的供体原子,胺N原子,磺酰胺基团的N原子和配位的水分子满足配位环境。[Mn(NO2ASAm)] -配合物的较低条件稳定性和磺酰胺基团的较低质子化常数导致在较高pH(<7.0)时复合物解离。然而,在[Mn(上DPASAm)]磺酰胺基团的质子化-落入生理学相关pH窗口并引起显著增加从弛豫- [R 1P = 3.8毫米-1小号-1在pH9.0到- [R 1P = 8.9毫米- 1个s –1在pH 4.0(10 MHz,25°C)下。
更新日期:2020-10-05
中文翻译:

含磺酰胺基的Mn2 +配合物,具有pH响应弛豫性。
我们含有本两个配体Ñ附接到乙基-4-(三氟甲基)苯磺酰胺基团或者是6,6' - (azanediylbis(亚甲基))吡啶二羧酸单元(H 3 DPASAm)或2,2' - (1, 4,7-三氮烷-1,4-二基)二乙酸大环平台(H 3 NO2ASAm)。这些配体经设计可在水溶液中与Mn 2+络合后提供pH依赖性弛豫响应。配体的质子化常数和Mn 2+络合物的稳定性常数是通过分光光度法补充的电位滴定法测定的。配体的磺酰胺基团的去质子化的特征在于log K i的质子化常数对于DPASAm 3–和HNO2ASAm 2–,H分别为10.36和10.59 。由于带负电的磺酰胺基团与金属离子的配位,在Mn 2+存在下,这些值急剧下降至log K i H = 6.43和5.42 。[Mn(DPASAm)] -中较高的log K i H值与七配位络合物的形成有关,而[Mn(NO2ASAm)] -中金属离子为六配位。Na [Mn(DPASAm)(H 2 O)] · 2H 2的X射线晶体结构O证实形成七配位配合物,其中两个吡啶甲酸基团的供体原子,胺N原子,磺酰胺基团的N原子和配位的水分子满足配位环境。[Mn(NO2ASAm)] -配合物的较低条件稳定性和磺酰胺基团的较低质子化常数导致在较高pH(<7.0)时复合物解离。然而,在[Mn(上DPASAm)]磺酰胺基团的质子化-落入生理学相关pH窗口并引起显著增加从弛豫- [R 1P = 3.8毫米-1小号-1在pH9.0到- [R 1P = 8.9毫米- 1个s –1在pH 4.0(10 MHz,25°C)下。


























 京公网安备 11010802027423号
京公网安备 11010802027423号