当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Questioning the Affinity of Electrophilic Astatine for Sulfur-containing Compounds: Unexpected Bindings Revealed.
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2020-09-22 , DOI: 10.1021/acs.inorgchem.0c01553 Fadel Bassal 1 , Julie Champion 2 , Sylvain Pardoue 1, 2 , Mahamadou Seydou 3 , Andrea Sabatié-Gogova 2 , David Deniaud 1 , Jean-Yves Le Questel 1 , Gilles Montavon 2 , Nicolas Galland 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2020-09-22 , DOI: 10.1021/acs.inorgchem.0c01553 Fadel Bassal 1 , Julie Champion 2 , Sylvain Pardoue 1, 2 , Mahamadou Seydou 3 , Andrea Sabatié-Gogova 2 , David Deniaud 1 , Jean-Yves Le Questel 1 , Gilles Montavon 2 , Nicolas Galland 1
Affiliation
|
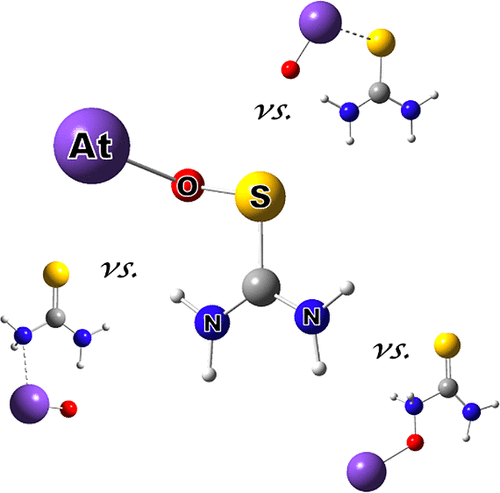
The affinity of AtO+ for around 20 model ligands (L), carrying functionalized oxygen, sulfur, and nitrogen atoms, has been assessed through a combined experimental and theoretical methodology. Significant equilibrium constants (KL ∼ 104) have been measured for sulfur-containing compounds, in agreement with the previously highlighted, relatively stable radiolabeling of SH-containing proteins with 211At. Conversely, no interaction occurs in the aqueous phase for their oxygenated counterparts, but higher affinities (KL > 106) have been determined for nitrogen-based ligands, including aromatic nitrogen heterocycles. The quantum mechanical calculations definitively ruled out any rationale based on either the metallic character of astatine or its guessed softness; the favored interactions all involve specifically the oxygen atom of AtO+, leading to the formation of covalent O–S or O–C single bonds.
中文翻译:

质疑亲电子A对含硫化合物的亲和力:揭示了意外的结合。
通过组合的实验和理论方法,评估了AtO +对约20个带有官能化氧,硫和氮原子的模型配体(L)的亲和力。显著平衡常数(ķ大号〜10 4)已经被测量含硫化合物,在协议与先前突出显示的,相对稳定的含有SH基的蛋白与放射性标记211在。相反,对于含氧化合物,在水相中没有相互作用,但亲和力更高(K L > 10 6已经确定了基于氮的配体,包括芳族氮杂环。量子力学计算绝对地基于on的金属特性或其推测的柔软度排除了任何理由。所有有利的相互作用都特别涉及AtO +的氧原子,从而导致形成共价的OS或OC单键。
更新日期:2020-10-05
中文翻译:

质疑亲电子A对含硫化合物的亲和力:揭示了意外的结合。
通过组合的实验和理论方法,评估了AtO +对约20个带有官能化氧,硫和氮原子的模型配体(L)的亲和力。显著平衡常数(ķ大号〜10 4)已经被测量含硫化合物,在协议与先前突出显示的,相对稳定的含有SH基的蛋白与放射性标记211在。相反,对于含氧化合物,在水相中没有相互作用,但亲和力更高(K L > 10 6已经确定了基于氮的配体,包括芳族氮杂环。量子力学计算绝对地基于on的金属特性或其推测的柔软度排除了任何理由。所有有利的相互作用都特别涉及AtO +的氧原子,从而导致形成共价的OS或OC单键。



























 京公网安备 11010802027423号
京公网安备 11010802027423号