当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Fast purification of recombinant monomeric amyloid-β from E. coli and amyloid-β-mCherry aggregates from mammalian cells.
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2020-09-22 , DOI: 10.1021/acschemneuro.0c00300 Amberley D Stephens 1 , Meng Lu 1 , Ana Fernandez-Villegas 1 , Gabriele S Kaminski Schierle 1
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2020-09-22 , DOI: 10.1021/acschemneuro.0c00300 Amberley D Stephens 1 , Meng Lu 1 , Ana Fernandez-Villegas 1 , Gabriele S Kaminski Schierle 1
Affiliation
|
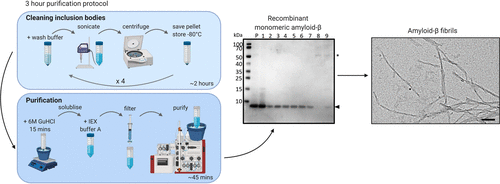
The Alzheimer’s disease related peptide, Amyloid-beta (Aβ)1–40 and 1–42, has proven difficult to be purified as a recombinant monomeric protein due its expression in E. coli leading to the formation of insoluble inclusion bodies and its tendency to quickly form insoluble aggregates. A vast array of methods have been used so far, yet many have pitfalls, such as the use of tags for ease of Aβ isolation, the formation of Aβ multimers within the time frame of extraction, or the need to reconstitute Aβ from a freeze–dried state. Here, we present a rapid protocol to produce highly pure and monomeric recombinant Aβ using a one-step ion exchange purification method and to label the peptide using a maleimide dye. The washing, solubilization, and purification steps take only 3 h. We also present a protocol for the isolation of Aβ-mCherry from mammalian cells.
中文翻译:

从大肠杆菌中快速纯化重组单体淀粉样蛋白-β,从哺乳动物细胞中快速纯化淀粉样蛋白-β-mCherry 聚集体。
阿尔茨海默病相关肽,β-淀粉样蛋白 (Aβ)1-40 和 1-42,由于其在大肠杆菌中的表达,已被证明难以作为重组单体蛋白纯化导致不溶性包涵体的形成及其迅速形成不溶性聚集体的趋势。迄今为止,已经使用了大量的方法,但许多方法都存在缺陷,例如使用标签以方便 Aβ 分离、在提取时间范围内形成 Aβ 多聚体,或者需要从冷冻中重建 Aβ——干燥状态。在这里,我们提出了一种快速协议,使用一步离子交换纯化方法生产高纯度和单体重组 Aβ,并使用马来酰亚胺染料标记肽。洗涤、溶解和纯化步骤仅需 3 小时。我们还提出了从哺乳动物细胞中分离 Aβ-mCherry 的协议。
更新日期:2020-10-21
中文翻译:

从大肠杆菌中快速纯化重组单体淀粉样蛋白-β,从哺乳动物细胞中快速纯化淀粉样蛋白-β-mCherry 聚集体。
阿尔茨海默病相关肽,β-淀粉样蛋白 (Aβ)1-40 和 1-42,由于其在大肠杆菌中的表达,已被证明难以作为重组单体蛋白纯化导致不溶性包涵体的形成及其迅速形成不溶性聚集体的趋势。迄今为止,已经使用了大量的方法,但许多方法都存在缺陷,例如使用标签以方便 Aβ 分离、在提取时间范围内形成 Aβ 多聚体,或者需要从冷冻中重建 Aβ——干燥状态。在这里,我们提出了一种快速协议,使用一步离子交换纯化方法生产高纯度和单体重组 Aβ,并使用马来酰亚胺染料标记肽。洗涤、溶解和纯化步骤仅需 3 小时。我们还提出了从哺乳动物细胞中分离 Aβ-mCherry 的协议。



























 京公网安备 11010802027423号
京公网安备 11010802027423号