当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
How Mutations perturb γ-Secretase active site studied by Free Energy Simulations.
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2020-09-22 , DOI: 10.1021/acschemneuro.0c00440 Shu-Yu Chen 1 , Martin Zacharias 1
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2020-09-22 , DOI: 10.1021/acschemneuro.0c00440 Shu-Yu Chen 1 , Martin Zacharias 1
Affiliation
|
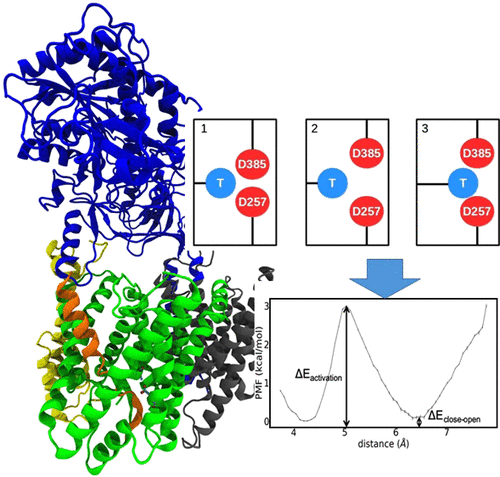
γ-Secretase is involved in processing of the amyloid precursor protein (APP) and generation of short Aβ peptides that may play a key role in neurodegenerative diseases such as Alzheimer’s disease (AD). Several mutations in γ-secretase influence its activity, resulting in early AD onset (Familial AD or FAD mutations). The molecular details of how mutations, not located close to the active site, can affect enzyme activity is not understood. In molecular dynamics simulations of γ-secretase in the absence of substrate (apo), we identified two active site conformational states characterized by a direct contact between catalytic Asp residues (closed state) and an open water-bridged state. In the presence of substrate, only conformations compatible with the open active site geometry are accessible. Systematic free energy simulations on wild type and FAD mutations indicate a free energy difference between closed and open states that is significantly modulated by FAD mutations and correlates with the corresponding experimental activity. For mutations with reduced activity, an increased penalty for open-state transitions was found. Only for two mutations located at the active site a direct perturbation of the open-state geometry was observed that could directly explain the drop of enzyme activity. The simulations suggest that modulation of the closed/open equilibrium and perturbation of the open (active) catalytic geometry are possible mechanisms of how FAD mutations affect γ-secretase activity. The results also offer an explanation for the experimental finding that FAD mutations, although not located at the interface to the substrate, mainly destabilize the enzyme–substrate complex.
中文翻译:

自由能量模拟研究了突变如何扰动γ-分泌酶活性位点。
γ分泌酶是参与短A的淀粉样前体蛋白(APP)和生成的处理β在神经退行性疾病(例如阿尔茨海默氏病(AD))中可能起关键作用的多肽。γ-分泌酶中的几个突变会影响其活性,导致AD早期发作(家族性AD或FAD突变)。目前尚不清楚分子的细节,即不位于活性位点附近的突变如何影响酶的活性。在不存在底物(apo)的情况下,γ-分泌酶的分子动力学模拟中,我们确定了两个活性位点构象状态,其特征在于催化性Asp残基之间直接接触(闭合状态)和开放水桥状态。在存在底物的情况下,仅可访问与开放活性位点几何形状相容的构象。对野生型和FAD突变的系统自由能模拟表明,封闭状态和开放状态之间的自由能差受FAD突变显着调节,并与相应的实验活动相关。对于活性降低的突变,发现增加了对开放状态转变的惩罚。仅对于位于活性位点的两个突变,观察到开放态几何结构的直接扰动可以直接解释酶活性的下降。该模拟表明,封闭/开放平衡的调节和开放(活性)催化几何结构的扰动是FAD突变如何影响γ-分泌酶活性的可能机制。结果也为实验发现提供了解释,即FAD突变虽然不位于与底物的界面,
更新日期:2020-10-21
中文翻译:

自由能量模拟研究了突变如何扰动γ-分泌酶活性位点。
γ分泌酶是参与短A的淀粉样前体蛋白(APP)和生成的处理β在神经退行性疾病(例如阿尔茨海默氏病(AD))中可能起关键作用的多肽。γ-分泌酶中的几个突变会影响其活性,导致AD早期发作(家族性AD或FAD突变)。目前尚不清楚分子的细节,即不位于活性位点附近的突变如何影响酶的活性。在不存在底物(apo)的情况下,γ-分泌酶的分子动力学模拟中,我们确定了两个活性位点构象状态,其特征在于催化性Asp残基之间直接接触(闭合状态)和开放水桥状态。在存在底物的情况下,仅可访问与开放活性位点几何形状相容的构象。对野生型和FAD突变的系统自由能模拟表明,封闭状态和开放状态之间的自由能差受FAD突变显着调节,并与相应的实验活动相关。对于活性降低的突变,发现增加了对开放状态转变的惩罚。仅对于位于活性位点的两个突变,观察到开放态几何结构的直接扰动可以直接解释酶活性的下降。该模拟表明,封闭/开放平衡的调节和开放(活性)催化几何结构的扰动是FAD突变如何影响γ-分泌酶活性的可能机制。结果也为实验发现提供了解释,即FAD突变虽然不位于与底物的界面,

























 京公网安备 11010802027423号
京公网安备 11010802027423号