当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemical N2 Reduction to Ammonia Using Single Au/Fe Atoms Supported on Nitrogen-Doped Porous Carbon
ACS Applied Energy Materials ( IF 6.4 ) Pub Date : 2020-09-23 , DOI: 10.1021/acsaem.0c01740 Sudhir K Sahoo 1 , Julian Heske 1, 2 , Markus Antonietti 2 , Qing Qin 2 , Martin Oschatz 2 , Thomas D Kühne 1, 3
ACS Applied Energy Materials ( IF 6.4 ) Pub Date : 2020-09-23 , DOI: 10.1021/acsaem.0c01740 Sudhir K Sahoo 1 , Julian Heske 1, 2 , Markus Antonietti 2 , Qing Qin 2 , Martin Oschatz 2 , Thomas D Kühne 1, 3
Affiliation
|
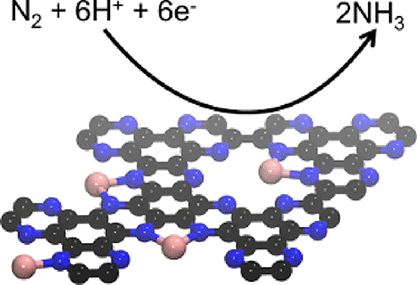
The electrochemical nitrogen reduction reaction (NRR) to ammonia (NH3) is a promising alternative route for an NH3 synthesis at ambient conditions to the conventional high temperature and pressure Haber–Bosch process without the need for hydrogen gas. Single metal ions or atoms are attractive candidates for the catalytic activation of non-reactive nitrogen (N2), and for future targeted improvement of NRR catalysts, it is of utmost importance to get detailed insights into structure-performance relationships and mechanisms of N2 activation in such structures. Here, we report density functional theory studies on the NRR catalyzed by single Au and Fe atoms supported in graphitic C2N materials. Our results show that the metal atoms present in the structure of C2N are the reactive sites, which catalyze the aforesaid reaction by strong adsorption and activation of N2. We further demonstrate that a lower onset electrode potential is required for Fe–C2N than for Au–C2N. Thus, Fe–C2N is theoretically predicted to be a potentially better NRR catalyst at ambient conditions than Au–C2N owing to the larger adsorption energy of N2 molecules. Furthermore, we have experimentally shown that single sites of Au and Fe supported on nitrogen-doped porous carbon are indeed active NRR catalysts. However, in contrast to our theoretical results, the Au-based catalyst performed slightly better with a Faradaic efficiency (FE) of 10.1% than the Fe-based catalyst with an FE of 8.4% at −0.2 V vs. RHE. The DFT calculations suggest that this difference is due to the competitive hydrogen evolution reaction and higher desorption energy of ammonia.
中文翻译:

使用氮掺杂多孔碳负载的单个 Au/Fe 原子将 N2 电化学还原为氨
电化学氮还原反应 (NRR) 生成氨 (NH 3 ) 是在环境条件下合成 NH 3的有前途的替代路线,可替代传统的高温高压 Haber–Bosch 工艺,无需氢气。单一金属离子或原子是非活性氮 (N 2 ) 催化活化的有吸引力的候选者,并且对于未来 NRR 催化剂的有针对性的改进,对 N 2的结构-性能关系和机制进行详细了解至关重要在这样的结构中激活。在这里,我们报告了由石墨 C 2中负载的单个 Au 和 Fe 原子催化的 NRR 的密度泛函理论研究N个材料。我们的结果表明,C 2 N结构中存在的金属原子是反应位点,通过对N 2的强吸附和活化催化上述反应。我们进一步证明,与 Au-C 2 N相比,Fe-C 2 N需要较低的起始电极电位。因此,理论上预测 Fe-C 2 N 在环境条件下可能是比 Au-C 2更好的 NRR 催化剂N由于N 2吸附能较大分子。此外,我们已经通过实验表明,负载在氮掺杂多孔碳上的 Au 和 Fe 单中心确实是活性 NRR 催化剂。然而,与我们的理论结果相反,Au 基催化剂的法拉第效率 (FE) 为 10.1%,略好于 Fe 基催化剂,在 -0.2 V vs.RHE 时的 FE 为 8.4%。DFT 计算表明,这种差异是由于竞争性析氢反应和较高的氨解吸能量。
更新日期:2020-10-26
中文翻译:

使用氮掺杂多孔碳负载的单个 Au/Fe 原子将 N2 电化学还原为氨
电化学氮还原反应 (NRR) 生成氨 (NH 3 ) 是在环境条件下合成 NH 3的有前途的替代路线,可替代传统的高温高压 Haber–Bosch 工艺,无需氢气。单一金属离子或原子是非活性氮 (N 2 ) 催化活化的有吸引力的候选者,并且对于未来 NRR 催化剂的有针对性的改进,对 N 2的结构-性能关系和机制进行详细了解至关重要在这样的结构中激活。在这里,我们报告了由石墨 C 2中负载的单个 Au 和 Fe 原子催化的 NRR 的密度泛函理论研究N个材料。我们的结果表明,C 2 N结构中存在的金属原子是反应位点,通过对N 2的强吸附和活化催化上述反应。我们进一步证明,与 Au-C 2 N相比,Fe-C 2 N需要较低的起始电极电位。因此,理论上预测 Fe-C 2 N 在环境条件下可能是比 Au-C 2更好的 NRR 催化剂N由于N 2吸附能较大分子。此外,我们已经通过实验表明,负载在氮掺杂多孔碳上的 Au 和 Fe 单中心确实是活性 NRR 催化剂。然而,与我们的理论结果相反,Au 基催化剂的法拉第效率 (FE) 为 10.1%,略好于 Fe 基催化剂,在 -0.2 V vs.RHE 时的 FE 为 8.4%。DFT 计算表明,这种差异是由于竞争性析氢反应和较高的氨解吸能量。



























 京公网安备 11010802027423号
京公网安备 11010802027423号