当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Influence of Amide Connectivity on the Hydrogen Bond Directed Self-Assembly of [n.n]Paracyclophanes.
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2020-09-23 , DOI: 10.1002/chem.202003909 Will R Henderson 1 , Ajeet Kumar 1 , Khalil A Abboud 1 , Ronald K Castellano 1
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2020-09-23 , DOI: 10.1002/chem.202003909 Will R Henderson 1 , Ajeet Kumar 1 , Khalil A Abboud 1 , Ronald K Castellano 1
Affiliation
|
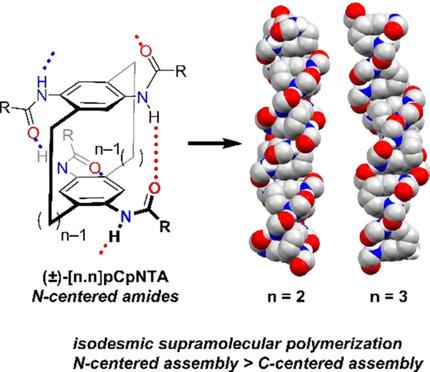
Reported here is the synthesis and self‐assembly characterization of [n.n]paracyclophanes ([n.n]pCps, n=2, 3) equipped with anilide hydrogen bonding units. These molecules differ from previous self‐assembling [n.n]paracyclophanes ([n.n]pCps) in the connectivity of their amide hydrogen bonding units (C‐centered/carboxamide vs. N‐centered/anilide). This subtle change results in a ≈30‐fold increase in the elongation constant for the [2.2]pCp‐4,7,12,15‐tetraanilide ([2.2]pCpNTA) compared to previously reported [2.2]pCp‐4,7,12,15‐tetracarboxamide ([2.2]pCpTA), and a ≈300‐fold increase in the elongation constant for the [3.3]pCp‐5,8,14,17‐tetraanilide ([3.3]pCpNTA) compared to previously reported [3.3]pCp‐5,8,14,17‐tetracarboxamide ([3.3]pCpTA). The [n.n]pCpNTA monomers also represent the reversal of a previously reported trend in solution‐phase assembly strength when comparing [2.2]pCpTA and [3.3]pCpTA monomers. The origins of the assembly differences are geometric changes in the association between [n.n]pCpNTA monomers—revealed by computations and X‐ray crystallography—resulting in a more favorable slipped stacking of the intermolecular π‐surfaces ([n.n]pCpNTA vs. [n.n]pCpTA), and a more complementary H‐bonding geometry ([3.3]pCpNTA vs. [2.2]pCpNTA).
中文翻译:

酰胺连接性对[nn]环芳烃氢键定向自组装的影响。
此处报道的是具有苯胺氢键单元的[nn]对环环烷([nn] pCps,n = 2,3)的合成和自组装表征。这些分子在酰胺氢键单元(C中心/羧酰胺vs. N中心/苯胺)的连接性方面与以前的自组装[nn]对环环烷([nn] pCps)不同。与之前报道的[2.2] pCp-4,7相比,这种微妙的变化导致[2.2] pCp-4,7,12,15-四苯胺([2.2] pCpNTA)的伸长常数增加了约30倍,12,15-四甲酰胺([2.2] pCpTA),与以前报道的[3.3] pCp-5,8,14 ,[3.3] pCp-5,8,14,17-四苯胺([3.3] pCpNTA)的伸长常数相比,增加了约300倍, 17-四甲酰胺([3.3] pCpTA)。当比较[2.2] pCpTA和[3.3] pCpTA单体时,[nn] pCpNTA单体也代表了先前报道的溶液相组装强度趋势的逆转。组装差异的根源是[nn] pCpNTA单体之间的缔合中的几何变化(通过计算和X射线晶体学揭示),导致分子间π表面([nn] pCpNTA]滑移堆积更有利与[nn] pCpTA相比),以及更互补的H键几何形状[[3.3] pCpNTA与[2.2] pCpNTA)。
更新日期:2020-09-23
中文翻译:

酰胺连接性对[nn]环芳烃氢键定向自组装的影响。
此处报道的是具有苯胺氢键单元的[nn]对环环烷([nn] pCps,n = 2,3)的合成和自组装表征。这些分子在酰胺氢键单元(C中心/羧酰胺vs. N中心/苯胺)的连接性方面与以前的自组装[nn]对环环烷([nn] pCps)不同。与之前报道的[2.2] pCp-4,7相比,这种微妙的变化导致[2.2] pCp-4,7,12,15-四苯胺([2.2] pCpNTA)的伸长常数增加了约30倍,12,15-四甲酰胺([2.2] pCpTA),与以前报道的[3.3] pCp-5,8,14 ,[3.3] pCp-5,8,14,17-四苯胺([3.3] pCpNTA)的伸长常数相比,增加了约300倍, 17-四甲酰胺([3.3] pCpTA)。当比较[2.2] pCpTA和[3.3] pCpTA单体时,[nn] pCpNTA单体也代表了先前报道的溶液相组装强度趋势的逆转。组装差异的根源是[nn] pCpNTA单体之间的缔合中的几何变化(通过计算和X射线晶体学揭示),导致分子间π表面([nn] pCpNTA]滑移堆积更有利与[nn] pCpTA相比),以及更互补的H键几何形状[[3.3] pCpNTA与[2.2] pCpNTA)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号