当前位置:
X-MOL 学术
›
Adv. Theory Simul.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Understanding the Dehydrogenation Pathways of Ammonium Octahydrotriborate (NH4B3H8) by Molecular Dynamics Simulations with the Reactive Force Field (ReaxFF)
Advanced Theory and Simulations ( IF 3.3 ) Pub Date : 2020-09-04 , DOI: 10.1002/adts.202000139 Peng Gao 1 , Jie Zhang 2, 3
Advanced Theory and Simulations ( IF 3.3 ) Pub Date : 2020-09-04 , DOI: 10.1002/adts.202000139 Peng Gao 1 , Jie Zhang 2, 3
Affiliation
|
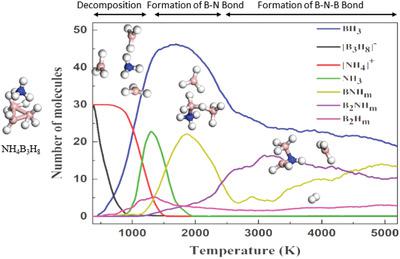
Ammonium octahydrotriborate (NH4B3H8) is a potential candidate for hydrogen storage, due to its chemical stability and high hydrogen content. To systematically investigate its initial decomposition and dehydrogenation pathways, molecular dynamics simulations with a reactive force field are conducted. Both temperature ramping and the canonical ensemble simulations are carried out. A compositional analysis for the simulated systems is also applied to monitor the possible intermediate products during its decomposition and dehydrogenation processes. At around 1000 K, the molecular hydrogen is released via inter‐molecular interaction between B‐H and N‐H . It is also noticed that the formed NH3 and BH3 can react to generate BN bonds; as the temperature further increases, the isomers with a skeleton of BNB are observed, and the corresponding dehydrogenation is also increased. The present work provides detailed pictures of the decomposition and dehydrogenation pathways for NH4B3H8.
中文翻译:

通过具有反应力场(ReaxFF)的分子动力学模拟了解八氢三硼酸铵(NH4B3H8)的脱氢途径
八氢三硼酸铵(NH 4 B 3 H 8)由于其化学稳定性和高氢含量,是潜在的储氢候选物。为了系统地研究其初始分解和脱氢途径,进行了具有反作用力场的分子动力学模拟。进行温度上升和规范的集成仿真。模拟系统的成分分析还用于监控其分解和脱氢过程中可能的中间产物。在大约1000 K时,氢原子通过B–H之间的分子间相互作用释放 和N‐H 。还注意到形成的NH 3和BH 3可以反应生成B BN键;随着温度进一步升高,以B的骨架异构体 Ñ B被观察到,并且相应的脱氢也增加。本工作提供了NH 4 B 3 H 8分解和脱氢途径的详细图片。
更新日期:2020-10-05
中文翻译:

通过具有反应力场(ReaxFF)的分子动力学模拟了解八氢三硼酸铵(NH4B3H8)的脱氢途径
八氢三硼酸铵(NH 4 B 3 H 8)由于其化学稳定性和高氢含量,是潜在的储氢候选物。为了系统地研究其初始分解和脱氢途径,进行了具有反作用力场的分子动力学模拟。进行温度上升和规范的集成仿真。模拟系统的成分分析还用于监控其分解和脱氢过程中可能的中间产物。在大约1000 K时,氢原子通过B–H之间的分子间相互作用释放 和N‐H 。还注意到形成的NH 3和BH 3可以反应生成B BN键;随着温度进一步升高,以B的骨架异构体 Ñ B被观察到,并且相应的脱氢也增加。本工作提供了NH 4 B 3 H 8分解和脱氢途径的详细图片。


























 京公网安备 11010802027423号
京公网安备 11010802027423号