当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Simultaneous Inhibition of SIRT2 Deacetylase and Defatty-Acylase Activities via a PROTAC Strategy
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2020-09-21 , DOI: 10.1021/acsmedchemlett.0c00423 Jun Young Hong 1 , Hui Jing 1 , Ian Robert Price 1 , Ji Cao 1 , Jessica Jingyi Bai 1 , Hening Lin 1, 2
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2020-09-21 , DOI: 10.1021/acsmedchemlett.0c00423 Jun Young Hong 1 , Hui Jing 1 , Ian Robert Price 1 , Ji Cao 1 , Jessica Jingyi Bai 1 , Hening Lin 1, 2
Affiliation
|
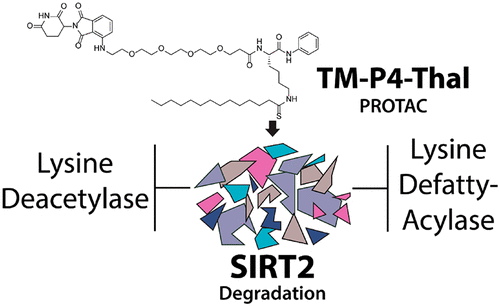
As a member of the sirtuin family of enzymes, SIRT2 promotes tumor growth and regulates various biological pathways through lysine deacetylation and defatty-acylation. In the past few years, many SIRT2-selective small molecule inhibitors have been developed, but none have demonstrated simultaneous inhibition of both SIRT2 activities in cells. To further scrutinize the physiological importance and significance of SIRT2 deacetylase and defatty-acylase activities, small molecules that can selectively inhibit both activities of SIRT2 in living cells are needed. Here, we have applied the Proteolysis Targeting Chimera (PROTAC) strategy and synthesized a new SIRT2 inhibitor (TM-P4-Thal) to degrade SIRT2 selectively, which led to simultaneous inhibition of its deacetylase and defatty-acylase activities in living cells. Additionally, this compound exemplifies the advantage of the PROTAC strategy that allows complete eradication of an enzyme and its activity in biological settings.
中文翻译:

通过 PROTAC 策略同时抑制 SIRT2 脱乙酰酶和脱脂酰化酶活性
作为 Sirtuin 酶家族的一员,SIRT2 通过赖氨酸脱乙酰化和脱脂酰化促进肿瘤生长并调节各种生物途径。在过去的几年中,已经开发了许多 SIRT2 选择性小分子抑制剂,但没有一种能同时抑制细胞中的两种 SIRT2 活性。为了进一步审查 SIRT2 脱乙酰酶和脱脂酰化酶活性的生理重要性和意义,需要能够选择性抑制活细胞中 SIRT2 两种活性的小分子。在这里,我们应用了蛋白水解靶向嵌合体 (PROTAC) 策略并合成了一种新的 SIRT2 抑制剂 (TM-P4-Thal) 来选择性降解 SIRT2,从而同时抑制其在活细胞中的脱乙酰酶和脱脂酰化酶活性。此外,
更新日期:2020-11-12
中文翻译:

通过 PROTAC 策略同时抑制 SIRT2 脱乙酰酶和脱脂酰化酶活性
作为 Sirtuin 酶家族的一员,SIRT2 通过赖氨酸脱乙酰化和脱脂酰化促进肿瘤生长并调节各种生物途径。在过去的几年中,已经开发了许多 SIRT2 选择性小分子抑制剂,但没有一种能同时抑制细胞中的两种 SIRT2 活性。为了进一步审查 SIRT2 脱乙酰酶和脱脂酰化酶活性的生理重要性和意义,需要能够选择性抑制活细胞中 SIRT2 两种活性的小分子。在这里,我们应用了蛋白水解靶向嵌合体 (PROTAC) 策略并合成了一种新的 SIRT2 抑制剂 (TM-P4-Thal) 来选择性降解 SIRT2,从而同时抑制其在活细胞中的脱乙酰酶和脱脂酰化酶活性。此外,


























 京公网安备 11010802027423号
京公网安备 11010802027423号