当前位置:
X-MOL 学术
›
J. Ind. Eng. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and luminescence properties of β-NaRE0.95Eu0.05F4 (RE = Y, Lu)
Journal of Industrial and Engineering Chemistry ( IF 6.1 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.jiec.2020.09.008 Illariia A. Razumkova , Alexander E. Sedykh , Yuriy G. Denisenko , Klaus Müller-Buschbaum
Journal of Industrial and Engineering Chemistry ( IF 6.1 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.jiec.2020.09.008 Illariia A. Razumkova , Alexander E. Sedykh , Yuriy G. Denisenko , Klaus Müller-Buschbaum
|
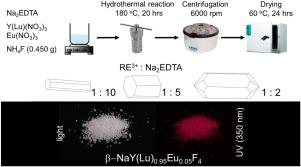
Abstract On the base of β-NaYF4 and β-NaLuF4 compounds, optimal synthesis methods were selected to obtain highly crystalline Eu3+-doped compounds in the hexagonal (β) structure. The compounds β-NaY0.95Eu0.05F4 and β-NaLu0.95Eu0.05F4 were synthesized by hydrothermal synthesis. The concentration of Na2EDTA introduced into the reaction system as a modifier affects the shape and size of the product from elongated hexagonal prisms to quartz-like crystals. The thermal behavior of the compounds has been studied; the temperature of the polymorphic transition from hexagonal to cubic structure is 680 °C for β-NaY0.95Eu0.05F4 and 624 °C for β-NaY0.95Eu0.05F4, the melting point of α-NaRE0.95Eu0.05F4 is about 900 °C. Under excitation with UV-light of λ = 393 nm, β-NaY0.95Eu0.05F4 and β-NaLu0.95Eu0.05F4 show the emission lines of direct Eu3+ f-f transitions, with the presence of the emission from higher Eu3+ excited states (up to 5H3) in both samples, which is responsible for the more orange color of emission instead of the usual red for Eu3+. Emission decay times are slightly longer for β-NaY0.95Eu0.05F4 than for β-NaLu0.95Eu0.05F4, which is the result of a bigger unit cell for the former, leading to longer interatomic distances between Eu3+ ions in the lattice. The luminescence mechanisms for the doped lanthanide ions were thoroughly analyzed.
中文翻译:

β-NaRE0.95Eu0.05F4 (RE = Y, Lu)的合成及发光特性
摘要 在β-NaYF4 和β-NaLuF4 化合物的基础上,选择最佳合成方法,获得高度结晶的六方(β) 结构的Eu3+ 掺杂化合物。采用水热法合成了化合物β-NaY0.95Eu0.05F4和β-NaLu0.95Eu0.05F4。作为改性剂引入反应系统的 Na2EDTA 的浓度会影响产品的形状和尺寸,从细长的六棱柱到类石英晶体。研究了化合物的热行为;β-NaY0.95Eu0.05F4的多晶型转变温度为680℃,β-NaY0.95Eu0.05F4为624℃,α-NaRE0.95Eu0.05F4的熔点约为900 ℃。在 λ = 393 nm 的紫外光激发下,β-NaY0.95Eu0.05F4 和 β-NaLu0.95Eu0.05F4 显示出直接 Eu3+ ff 跃迁的发射线,两个样品中都存在来自更高 Eu3+ 激发态(高达 5H3)的发射,这导致发射的颜色更橙色,而不是 Eu3+ 通常的红色。β-NaY0.95Eu0.05F4 的发射衰减时间比 β-NaLu0.95Eu0.05F4 稍长,这是因为前者的晶胞更大,导致晶格中 Eu3+ 离子之间的原子间距离更长。对掺杂的镧系元素离子的发光机制进行了彻底的分析。导致晶格中 Eu3+ 离子之间的原子间距离更长。对掺杂的镧系元素离子的发光机制进行了彻底的分析。导致晶格中 Eu3+ 离子之间的原子间距离更长。对掺杂的镧系元素离子的发光机制进行了彻底的分析。
更新日期:2020-12-01
中文翻译:

β-NaRE0.95Eu0.05F4 (RE = Y, Lu)的合成及发光特性
摘要 在β-NaYF4 和β-NaLuF4 化合物的基础上,选择最佳合成方法,获得高度结晶的六方(β) 结构的Eu3+ 掺杂化合物。采用水热法合成了化合物β-NaY0.95Eu0.05F4和β-NaLu0.95Eu0.05F4。作为改性剂引入反应系统的 Na2EDTA 的浓度会影响产品的形状和尺寸,从细长的六棱柱到类石英晶体。研究了化合物的热行为;β-NaY0.95Eu0.05F4的多晶型转变温度为680℃,β-NaY0.95Eu0.05F4为624℃,α-NaRE0.95Eu0.05F4的熔点约为900 ℃。在 λ = 393 nm 的紫外光激发下,β-NaY0.95Eu0.05F4 和 β-NaLu0.95Eu0.05F4 显示出直接 Eu3+ ff 跃迁的发射线,两个样品中都存在来自更高 Eu3+ 激发态(高达 5H3)的发射,这导致发射的颜色更橙色,而不是 Eu3+ 通常的红色。β-NaY0.95Eu0.05F4 的发射衰减时间比 β-NaLu0.95Eu0.05F4 稍长,这是因为前者的晶胞更大,导致晶格中 Eu3+ 离子之间的原子间距离更长。对掺杂的镧系元素离子的发光机制进行了彻底的分析。导致晶格中 Eu3+ 离子之间的原子间距离更长。对掺杂的镧系元素离子的发光机制进行了彻底的分析。导致晶格中 Eu3+ 离子之间的原子间距离更长。对掺杂的镧系元素离子的发光机制进行了彻底的分析。



























 京公网安备 11010802027423号
京公网安备 11010802027423号