Chemical Engineering Research and Design ( IF 3.9 ) Pub Date : 2020-09-22 , DOI: 10.1016/j.cherd.2020.09.021 Abhishek MS , Debasis Hazra , Gerry Steele , Sharmistha Pal
|
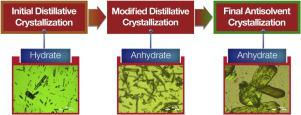
A case study highlighting the risk of generating undesired hydrate polymorph following seeded distillative crystallization of an active pharmaceutical ingredient (API) from aqueous alcoholic solution and a strategy to overcome the issue is presented. The API salt had two known crystalline forms, the desired anhydrate Form A, and hydrate Form B. Importance of water content at the time of seeding to minimize the risk of precipitation of From B is emphasized. A novel approach termed distillative pathway diagram (DPD) was developed to assess the risk of generation of Form B at larger scales. The application of this diagram to determine process parameters to address form issues during distillative crystallization is explained. Given the likely risk of Form B precipitation predicted by DPD, an alternate non-aqueous antisolvent crystallization process was developed that provided both form and particle size control.
中文翻译:

修改结晶工艺以解决API盐早期开发中的多晶型和粒径挑战
案例研究突出了从含酒精的溶液中进行活性药物成分(API)的晶种蒸馏结晶后生成不希望的水合物多晶型物的风险,并提出了解决该问题的策略。API盐具有两种已知的结晶形式,即所需的无水物形式A和水合物形式B。强调了播种时水分含量的重要性,以最大程度地减少B析出的风险。开发了一种称为蒸馏路径图(DPD)的新方法,以评估大规模生产B型的风险。解释了此图在确定工艺参数以解决蒸馏结晶过程中形式问题方面的应用。考虑到DPD预测的B型沉淀的可能风险,


























 京公网安备 11010802027423号
京公网安备 11010802027423号