Biochimie ( IF 3.9 ) Pub Date : 2020-09-22 , DOI: 10.1016/j.biochi.2020.09.013 Bhavna Maurya , Lionel Pochet , Johan Wouters , Melwin Colaço , Sandra Misquith
|
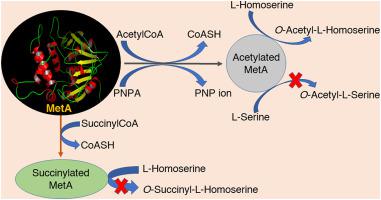
The metA (Rv3341) gene from Mycobacterium tuberculosis H37Rv strain encodes a homoserine-acetyltransferase (HAT) enzyme, also called MetA. This enzyme plays a key role in the biosynthetic pathway of methionine and is a potential target for the development of antimicrobial drugs. Purified MetA showed 40 kDa molecular mass on SDS-PAGE. Manual docking was performed with substrates acetyl-CoA, l-homoserine, and p-nitrophenylacetate using crystal structure coordinates of MetA (PDB ID 6PUX) from M. tuberculosis. Multiple sequence alignment indicated that catalytic triad residues Ser157, Asp320, His350 were conserved across species in acetyltransferases, esterases, and hydrolases. As a conserved pentapeptide, GXSMG belongs to α/β hydrolase superfamily and it shares similarity with esterases and hydrolases from different sources. Hydrolase activity of MetA was tested using (PNPA), N-acetylglycine, N-acetylmethionine and Phe-Gly as substrate. LC-MS confirmed that MetA possessed HAT activity, but no homoserine-succinyltransferase (HST) and serine-acetyltransferase (SAT) activities. Replacing acetyl-CoA with PNPA as acetyl group donor showed a drastic reduction in transferase activity, arising due to the interaction of R227 of the enzyme with PNPA. This could prevent the binding of the second substrate in the right orientation and results in the preferential transfer of the acetyl group to water, thus exhibiting hydrolase rather than transferase activity. In this paper, we report that MetA has both transferase and hydrolase activity depending on the correct orientation of the second substrate and the availability of the amino acids involved in enzyme-substrate interaction.
中文翻译:

结核分枝杆菌H37Rv菌株的MetA(Rv3341)表现出依赖底物的转移酶和水解酶活性双重作用
来自结核分枝杆菌H37Rv菌株的metA(Rv3341)基因编码高丝氨酸乙酰基转移酶(HAT)酶,也称为MetA。该酶在蛋氨酸的生物合成途径中起关键作用,并且是开发抗菌药物的潜在目标。纯化的MetA在SDS-PAGE上显示40 kDa的分子量。手动对接用基板乙酰辅酶A,执行升高丝氨酸,和p -nitrophenylacetate使用元(PDB ID 6PUX)的晶体结构坐标从结核分枝杆菌。多个序列比对表明,催化三联体残基Ser157,Asp320,His350在乙酰转移酶,酯酶和水解酶中跨物种保守。作为保守的五肽,GXSMG属于α/β水解酶超家族,与来自不同来源的酯酶和水解酶具有相似性。使用(PNPA),N-乙酰基甘氨酸,N-乙酰基蛋氨酸和Phe-Gly作为底物测试MetA的水解酶活性。LC-MS证实MetA具有HAT活性,但没有高丝氨酸-琥珀酰转移酶(HST)和丝氨酸-乙酰转移酶(SAT)活性。用PNPA代替乙酰辅酶A作为乙酰基供体,由于酶的R227与PNPA相互作用,导致转移酶活性急剧降低。这可以防止第二种底物以正确的方向结合,并导致乙酰基优先转移到水上,从而表现出水解酶而不是转移酶的活性。在本文中,我们报道了MetA具有转移酶和水解酶的活性,这取决于第二种底物的正确方向以及参与酶-底物相互作用的氨基酸的可用性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号