当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic Insights into the Oxidative Rearrangement Catalyzed by the Unprecedented Dioxygenase ChaP Involved in Chartreusin Biosynthesis.
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2020-09-20 , DOI: 10.1021/acs.inorgchem.0c01706 Xinyi Li 1 , Wenyou Zhu 2 , Yongjun Liu 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2020-09-20 , DOI: 10.1021/acs.inorgchem.0c01706 Xinyi Li 1 , Wenyou Zhu 2 , Yongjun Liu 1
Affiliation
|
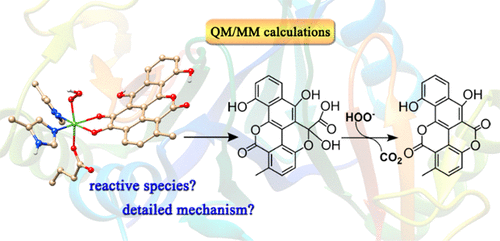
ChaP is a non-heme iron-dependent dioxygenase belonging to the vicinal oxygen chelate (VOC) enzyme superfamily that catalyzes the final α-pyrone ring formation in the biosynthesis of chartreusin. In contrast to other common dioxygenases, for example, 2,3-catechol dioxygenase which uses the dioxygen molecule as the oxidant, ChaP requires the flavin-activated oxygen (O22–) as the equivalent. Previous experiments showed that the ChaP-catalyzed ring rearrangement contains two successive C–C bond cleavages and one lactonization; however, the detailed reaction mechanism is unknown. In this work, on the basis of the recently obtained crystal structure of ChaP, the computational model was constructed and the catalytic mechanism of ChaP was explored by performing quantum mechanical/molecular mechanical (QM/MM) calculations. Our calculation results reveal that ChaP uses the proximal oxygen in iron-coordinated HOO– to attack the carbonyl carbon of the substrate, whereas the previous proposal that Asp49 acts as a base to deprotonate the iron-coordinated HOO– to generate O22– is unlikely. In the first stage reaction, owing to the coordination of the substrate with iron, the substrate is activated by accepting an electron from iron and the resulting oxy-radical intermediate formed by O–O cleavage can easily trigger the ring rearrangement. In the final decarboxylation, the phenolic anion of the substrate cooperatively accepts the proton of iron-coordinated HOO– to facilitate the attack of the distal oxygen, and the proton-coupled electron transfer (PCET) from the substrate to the FeIV═O plays a key role for the decarboxylation. These findings may provide useful information for understanding the ChaP-catalyzed oxidative rearrangement and other flavin-dependent non-heme dioxygenases.
中文翻译:

机械见解的空前的双氧合酶ChaP催化氧化黄绿素生物合成催化的氧化重排。
ChaP是属于血红素铁螯合(VOC)酶超家族的非血红素铁依赖性双加氧酶,可催化黄褐斑生物素在生物合成中的最终α-吡喃酮环形成。与其他常见的双加氧酶(例如,使用双加氧分子作为氧化剂的2,3-邻苯二酚双加氧酶)不同,ChaP需要黄素活化的氧(O 2 2–)等价物。先前的实验表明,ChaP催化的环重排包含两次连续的C–C键裂解和一次内酯化;但是,具体的反应机理尚不清楚。在这项工作中,在最近获得的ChaP晶体结构的基础上,通过进行量子力学/分子力学(QM / MM)计算,建立了计算模型并探索了ChaP的催化机理。我们的计算结果表明,ChaP使用铁配位的HOO中的近端氧-攻击底物的羰基碳,而先前的建议Asp49作为铁配位的HOO的质子化碱-生成O 2 2–不太可能。在第一阶段反应中,由于底物与铁的配位,底物通过接受铁中的电子而活化,并且通过O-O裂解形成的氧自由基中间体很容易触发环的重排。在最后的脱羧,在基板的酚醛阴离子协同接受铁协调HOO的质子-便于远侧氧的攻击,并且从基板的质子偶联电子转移(PCET)到Fe的IV = O戏剧脱羧的关键作用。这些发现可能为了解ChaP催化的氧化重排和其他黄素依赖性非血红素双加氧酶提供有用的信息。
更新日期:2020-10-05
中文翻译:

机械见解的空前的双氧合酶ChaP催化氧化黄绿素生物合成催化的氧化重排。
ChaP是属于血红素铁螯合(VOC)酶超家族的非血红素铁依赖性双加氧酶,可催化黄褐斑生物素在生物合成中的最终α-吡喃酮环形成。与其他常见的双加氧酶(例如,使用双加氧分子作为氧化剂的2,3-邻苯二酚双加氧酶)不同,ChaP需要黄素活化的氧(O 2 2–)等价物。先前的实验表明,ChaP催化的环重排包含两次连续的C–C键裂解和一次内酯化;但是,具体的反应机理尚不清楚。在这项工作中,在最近获得的ChaP晶体结构的基础上,通过进行量子力学/分子力学(QM / MM)计算,建立了计算模型并探索了ChaP的催化机理。我们的计算结果表明,ChaP使用铁配位的HOO中的近端氧-攻击底物的羰基碳,而先前的建议Asp49作为铁配位的HOO的质子化碱-生成O 2 2–不太可能。在第一阶段反应中,由于底物与铁的配位,底物通过接受铁中的电子而活化,并且通过O-O裂解形成的氧自由基中间体很容易触发环的重排。在最后的脱羧,在基板的酚醛阴离子协同接受铁协调HOO的质子-便于远侧氧的攻击,并且从基板的质子偶联电子转移(PCET)到Fe的IV = O戏剧脱羧的关键作用。这些发现可能为了解ChaP催化的氧化重排和其他黄素依赖性非血红素双加氧酶提供有用的信息。


























 京公网安备 11010802027423号
京公网安备 11010802027423号