当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An Overview of Water‐Mediated Alkyne Functionalization by Neighboring Group Participation of Carbonyl Groups
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-09-20 , DOI: 10.1002/adsc.202000826 Tapas R. Pradhan 1 , Jin Kyoon Park 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-09-20 , DOI: 10.1002/adsc.202000826 Tapas R. Pradhan 1 , Jin Kyoon Park 1
Affiliation
|
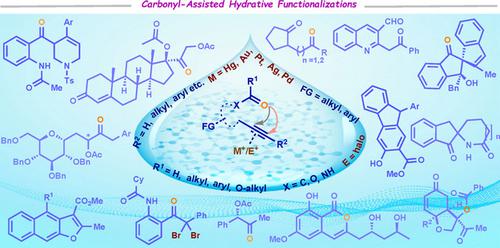
Reviewed herein, the progress employing the assistance of a neighboring carbonyl group for alkyne functionalization reactions that are mediated by water. Proper arrangement of these transformations is synthetically worthwhile because it will allow chemists to design straightforward synthetic routes for the construction of complex architectures with perfect regio‐ and chemoselectivity. Researchers must understand the mechanism before aiming a new idea based on these types of transformations as they proceed through a different way in comparison to the strategies that employ carbonyl groups as a directing group for C−H functionalization reactions. In general, an oxygen transposition event occurs in the presence of an alkyne activator. In an aim to expose the molecular diversity that can now be accessed employing intramolecular carbonyl assistance, all the precedents are organized systematically for the first time and can be expected to serve as a comprehensive reference work. Therefore, the key arrangement of this review is schemes accompanied by a concise explanatory text describing both advantages and limitations along with the mechanism. Different transformations are ordered in sections according to the chemical structure of the assisted groups. The overall objective of this review is to promote the application of carbonyl‐assisted reactions beyond the methodologies dedicated to the regioselective synthesis of carbonyl derivatives and hence, serving it as a valuable reaction archetype in modern‐day research for the synthesis of carbo‐ and heterocycles.
中文翻译:

羰基基团的相邻基团参与水介导的炔烃官能化概述
本文回顾了利用邻近羰基的辅助进行由水介导的炔官能化反应的进展。这些转换的正确安排在合成上是值得的,因为它将使化学家能够设计出简单的合成路线,以构建具有理想的区域选择性和化学选择性的复杂结构。与基于羰基作为CH官能化反应的导向基团的策略相比,研究人员必须以一种不同的方式进行研究,因此在基于这些类型的转化提出新思路之前,研究人员必须了解其机理。通常,氧转移事件在炔烃活化剂的存在下发生。为了揭示分子内羰基辅助下现在可以利用的分子多样性,所有的先例都是第一次系统地组织起来,有望作为一项全面的参考工作。因此,本文的主要安排是在方案中附以简洁的解释性文字,同时说明优点和缺点以及机制。根据辅助基团的化学结构,按部分对不同的转化进行排序。这篇综述的总体目标是促进羰基辅助反应的应用,超越专门用于羰基衍生物的区域选择性合成的方法,因此,在现代研究碳和杂环的合成中,它是有价值的反应原型。本文的主要安排是方案,并附有简要的解释性文字,描述优点和限制以及机制。根据辅助基团的化学结构,按部分对不同的转化进行排序。这篇综述的总体目标是促进羰基辅助反应的应用,超越专门用于羰基衍生物的区域选择性合成的方法,因此,在现代研究碳和杂环的合成中,它是有价值的反应原型。本文的主要安排是方案,并附有简要的解释性文字,描述优点和限制以及机制。根据辅助基团的化学结构,按部分对不同的转化进行排序。这篇综述的总体目标是促进羰基辅助反应的应用,超越专门用于羰基衍生物的区域选择性合成的方法,因此,在现代研究碳和杂环的合成中,它是有价值的反应原型。
更新日期:2020-11-19
中文翻译:

羰基基团的相邻基团参与水介导的炔烃官能化概述
本文回顾了利用邻近羰基的辅助进行由水介导的炔官能化反应的进展。这些转换的正确安排在合成上是值得的,因为它将使化学家能够设计出简单的合成路线,以构建具有理想的区域选择性和化学选择性的复杂结构。与基于羰基作为CH官能化反应的导向基团的策略相比,研究人员必须以一种不同的方式进行研究,因此在基于这些类型的转化提出新思路之前,研究人员必须了解其机理。通常,氧转移事件在炔烃活化剂的存在下发生。为了揭示分子内羰基辅助下现在可以利用的分子多样性,所有的先例都是第一次系统地组织起来,有望作为一项全面的参考工作。因此,本文的主要安排是在方案中附以简洁的解释性文字,同时说明优点和缺点以及机制。根据辅助基团的化学结构,按部分对不同的转化进行排序。这篇综述的总体目标是促进羰基辅助反应的应用,超越专门用于羰基衍生物的区域选择性合成的方法,因此,在现代研究碳和杂环的合成中,它是有价值的反应原型。本文的主要安排是方案,并附有简要的解释性文字,描述优点和限制以及机制。根据辅助基团的化学结构,按部分对不同的转化进行排序。这篇综述的总体目标是促进羰基辅助反应的应用,超越专门用于羰基衍生物的区域选择性合成的方法,因此,在现代研究碳和杂环的合成中,它是有价值的反应原型。本文的主要安排是方案,并附有简要的解释性文字,描述优点和限制以及机制。根据辅助基团的化学结构,按部分对不同的转化进行排序。这篇综述的总体目标是促进羰基辅助反应的应用,超越专门用于羰基衍生物的区域选择性合成的方法,因此,在现代研究碳和杂环的合成中,它是有价值的反应原型。


























 京公网安备 11010802027423号
京公网安备 11010802027423号