当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Selective Modification for Red‐Shifted Excitability: A Small Change in Structure, a Huge Change in Photochemistry
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2020-09-21 , DOI: 10.1002/chem.202003672 Yvonne Becker 1 , Sina Roth 2 , Maximilian Scheurer 3 , Andreas Jakob 1 , Daniel A Gacek 4 , Peter J Walla 4 , Andreas Dreuw 3 , Josef Wachtveitl 2 , Alexander Heckel 1
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2020-09-21 , DOI: 10.1002/chem.202003672 Yvonne Becker 1 , Sina Roth 2 , Maximilian Scheurer 3 , Andreas Jakob 1 , Daniel A Gacek 4 , Peter J Walla 4 , Andreas Dreuw 3 , Josef Wachtveitl 2 , Alexander Heckel 1
Affiliation
|
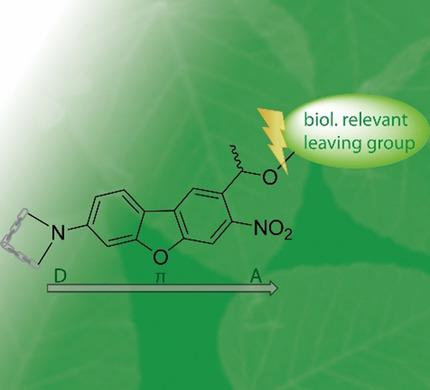
We developed three bathochromic, green‐light activatable, photolabile protecting groups based on a nitrodibenzofuran (NDBF) core with D‐π‐A push–pull structures. Variation of donor substituents (D) at the favored ring position enabled us to observe their impact on the photolysis quantum yields. Comparing our new azetidinyl‐NDBF (Az‐NDBF) photolabile protecting group with our earlier published DMA‐NDBF, we obtained insight into its excitation‐specific photochemistry. While the “two‐photon‐only” cage DMA‐NDBF was inert against one‐photon excitation (1PE) in the visible spectral range, we were able to efficiently release glutamic acid from azetidinyl‐NDBF with irradiation at 420 and 530 nm. Thus, a minimal change (a cyclization adding only one carbon atom) resulted in a drastically changed photochemical behavior, which enables photolysis in the green part of the spectrum.
中文翻译:

红移激发性的选择性修饰:结构的小变化,光化学的大变化
我们基于具有D-π-A推挽结构的硝基二苯并呋喃(NDBF)核,开发了三个可向红变色,可绿光激活的光不稳定保护基。供体取代基(D)在有利环位置的变化使我们能够观察到它们对光解量子产率的影响。将我们的新氮杂环丁烷基-NDBF(Az-NDBF)光不稳定保护基团与我们较早发布的DMA-NDBF进行比较,我们获得了其激发特异性光化学的见解。虽然“仅双光子”笼式DMA-NDBF在可见光谱范围内对单光子激发(1PE)呈惰性,但我们能够在420和530 nm的辐射下从氮杂环丁烷基-NDBF有效释放谷氨酸。因此,最小的变化(仅添加一个碳原子的环化反应)会导致光化学行为发生剧烈变化,
更新日期:2020-09-21
中文翻译:

红移激发性的选择性修饰:结构的小变化,光化学的大变化
我们基于具有D-π-A推挽结构的硝基二苯并呋喃(NDBF)核,开发了三个可向红变色,可绿光激活的光不稳定保护基。供体取代基(D)在有利环位置的变化使我们能够观察到它们对光解量子产率的影响。将我们的新氮杂环丁烷基-NDBF(Az-NDBF)光不稳定保护基团与我们较早发布的DMA-NDBF进行比较,我们获得了其激发特异性光化学的见解。虽然“仅双光子”笼式DMA-NDBF在可见光谱范围内对单光子激发(1PE)呈惰性,但我们能够在420和530 nm的辐射下从氮杂环丁烷基-NDBF有效释放谷氨酸。因此,最小的变化(仅添加一个碳原子的环化反应)会导致光化学行为发生剧烈变化,


























 京公网安备 11010802027423号
京公网安备 11010802027423号