Journal of Molecular Liquids ( IF 6 ) Pub Date : 2020-09-21 , DOI: 10.1016/j.molliq.2020.114365 Atsushi Matsuoka , Eiji Kamio , Hideto Matsuyama
|
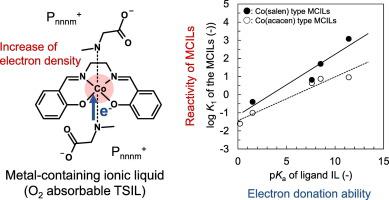
Metal-containing ionic liquids (MCILs) composed of a cobalt(II) Schiff base complex and ionic-liquid-based axial ligands (ligand ILs) are potential O2 absorbents. To determine the design criteria of MCILs with both highly selective O2 absorbability and low viscosity, the relationship between the physicochemical properties and chemical structure of the MCILs was investigated. The measurement of the amount of O2 absorbed in MCILs with various ligand ILs indicated that O2 reactivity is determined by the electron density of the Co atom of the MCILs. The electron density of the Co atom could be controlled by the σ electron donation ability of the ligand ILs. Moreover, the viscosity of the MCILs was strongly affected by the interaction among the MCIL molecules caused by the π electron system. This interaction was weakened by the equatorially coordinating Schiff base and the ligand ILs within the chemical structure. Therefore, to develop MCILs with both high O2 reactivity and low viscosity, suppression of the interaction caused by the π electron system without decreasing the electron density of the Co atom is important.
中文翻译:

配体结构对含金属离子液体的氧吸收能力和粘度的影响
由钴(II)席夫碱配合物和基于离子液体的轴向配体(配体IL)组成的含金属离子液体(MCIL)是潜在的O 2吸收剂。为了确定具有高选择性O 2吸收性和低粘度的MCIL的设计标准,研究了MCIL的理化性质与化学结构之间的关系。对具有各种配体IL的MCIL中吸收的O 2量的测量表明,O 2反应性由MCIL的Co原子的电子密度决定。Co原子的电子密度可以通过配体IL的σ电子给定能力来控制。此外,由π电子系统引起的MCIL分子之间的相互作用强烈影响MCIL的粘度。赤道配位的席夫碱和化学结构内的配体IL削弱了这种相互作用。因此,为了开发具有高O 2反应性和低粘度的MCIL,重要的是在不降低Co原子的电子密度的情况下抑制由π电子系统引起的相互作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号