Journal of CO2 Utilization ( IF 7.7 ) Pub Date : 2020-09-21 , DOI: 10.1016/j.jcou.2020.101313 Ning Rui , Kaihang Sun , Chenyang Shen , Chang-Jun Liu
|
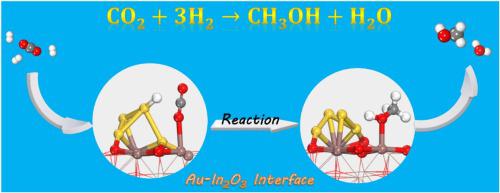
Density functional theoretical (DFT) study was conducted to investigate the feasibility of CO2 hydrogenation to methanol on an Au4/In2O3 model catalyst. A strong metal-support interaction is confirmed by the binding energy between Au4 cluster and In2O3 support, which is −5.31 eV. This causes the electron redistribution at the interfacial sites and leads to a positively charged Auδ+ cluster instead of metallic Au0. The positive oxidation state of Auδ+ cluster is originating from neighboring O atoms, i.e., the electron of Au cluster is transferred to adjacent O atom through Au O bond. This electron redistribution can activate Auδ+ cluster by generating electron depleted regions, which are active for H2 dissociation. The feasibility and reaction route of methanol synthesis from CO2 hydrogenation at Au-In2O3 interface is further examined to understand the effect of Au-In2O3 interaction on the catalytic performance. CO2 is activated at the interface, and formate is the key intermediate during the reaction. The rate-limiting step is hydroxyl spills over from Au cluster to In2O3 surface to release active Au site, with a reaction barrier of 0.95 eV. This can prevent hydroxyl from binding strongly with Au cluster and deactivate the catalyst. Compared with CH2OH intermediate, CH3O intermediate is more favorable in the final hydrogenation step. The DFT study suggests that the Au/In2O3 catalyst is a promising one for methanol synthesis from selective hydrogenation of CO2.
O bond. This electron redistribution can activate Auδ+ cluster by generating electron depleted regions, which are active for H2 dissociation. The feasibility and reaction route of methanol synthesis from CO2 hydrogenation at Au-In2O3 interface is further examined to understand the effect of Au-In2O3 interaction on the catalytic performance. CO2 is activated at the interface, and formate is the key intermediate during the reaction. The rate-limiting step is hydroxyl spills over from Au cluster to In2O3 surface to release active Au site, with a reaction barrier of 0.95 eV. This can prevent hydroxyl from binding strongly with Au cluster and deactivate the catalyst. Compared with CH2OH intermediate, CH3O intermediate is more favorable in the final hydrogenation step. The DFT study suggests that the Au/In2O3 catalyst is a promising one for methanol synthesis from selective hydrogenation of CO2.
中文翻译:

Au 4 / In 2 O 3催化剂用于CO 2加氢制甲醇的密度泛函理论研究:强金属-载体相互作用及其作用
进行了密度泛函理论(DFT)研究,以研究在Au 4 / In 2 O 3模型催化剂上将CO 2加氢成甲醇的可行性。Au 4团簇与In 2 O 3载体之间的结合能为-5.31 eV,证实了较强的金属-载体相互作用。这导致电子在界面部位重新分布,并导致带正电的Auδ +簇代替金属Au 0。Auδ+团簇的正氧化态源自相邻的O原子,即Au团簇的电子通过Au转移到相邻的O原子 O键。这种电子再分布可以通过产生对H 2分解具有活性的电子耗尽区来激活Auδ +团簇。进一步研究了Au-In 2 O 3界面上CO 2加氢合成甲醇的可行性和反应路线,以了解Au-In 2 O 3相互作用对催化性能的影响。CO 2在界面处被激活,甲酸是反应过程中的关键中间体。限速步骤是羟基从金簇向In 2 O 3溢出表面释放活性金位点,反应势垒为0.95 eV。这可以防止羟基牢固地与金簇结合并使催化剂失活。与CH 2 OH中间体相比,CH 3 O中间体在最终的氢化步骤中更有利。DFT研究表明,Au / In 2 O 3催化剂是用于CO 2选择性加氢合成甲醇的有前途的催化剂。
O键。这种电子再分布可以通过产生对H 2分解具有活性的电子耗尽区来激活Auδ +团簇。进一步研究了Au-In 2 O 3界面上CO 2加氢合成甲醇的可行性和反应路线,以了解Au-In 2 O 3相互作用对催化性能的影响。CO 2在界面处被激活,甲酸是反应过程中的关键中间体。限速步骤是羟基从金簇向In 2 O 3溢出表面释放活性金位点,反应势垒为0.95 eV。这可以防止羟基牢固地与金簇结合并使催化剂失活。与CH 2 OH中间体相比,CH 3 O中间体在最终的氢化步骤中更有利。DFT研究表明,Au / In 2 O 3催化剂是用于CO 2选择性加氢合成甲醇的有前途的催化剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号