当前位置:
X-MOL 学术
›
J. Alloys Compd.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development of the electrochemical performance of zinc via alloying with indium as anode for alkaline batteries application
Journal of Alloys and Compounds ( IF 6.2 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.jallcom.2020.157285 Mahmoud Elrouby , Hoda A. El –Shafy Shilkamy , A. Elsayed
Journal of Alloys and Compounds ( IF 6.2 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.jallcom.2020.157285 Mahmoud Elrouby , Hoda A. El –Shafy Shilkamy , A. Elsayed
|
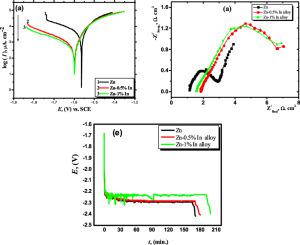
Abstract Zinc is one of the predominantly utilized metals in alkaline batteries. The addition of a trace amount of indium as an alloying element to zinc retards the rate of corrosion and promotes the sacrificial protection of zinc. The corrosion behavior of Zn and Zn–In alloy at various In content (0.5% and 1%, In/Zn mass fraction) in 6 M KOH solution was electrochemically studied. Tafel plots, electrochemical impedance spectroscopy (EIS), cyclic voltammetry (CV), and charge-discharge methods were all employed. The morphology, chemical composition, and phases of the corrosion layers formed on the surfaces of Zn and Zn–In alloys were thoroughly investigated utilizing scanning electron microscopy (SEM) equipped with an X-ray of dispersed energy (EADX) and X-ray diffraction (XRD), respectively. The polarization results revealed that the corrosion protection efficiency of the Zn–1%In alloy has the highest value of 97.3% at 45 °C. The steady-state of open circuit potential (Ecorr.) for the investigated alloys is shifted to a more negative value compared to that of zinc. This indicates that the alloying of zinc with indium has a positive effect on charge efficiency, suppression of hydrogen evolution reaction, and the capacitance. Moreover, the discharge time increases with the increase of the indium percentage in the bimetallic solid solution of Zn–In. The synthesized alloy is considered a promising material for long life alkaline batteries.
中文翻译:

通过与铟合金化锌作为碱性电池阳极的电化学性能的发展
摘要 锌是碱性电池中主要使用的金属之一。在锌中添加微量铟作为合金元素可减缓腐蚀速度并促进锌的牺牲保护。电化学研究了不同 In 含量(0.5% 和 1%,In/Zn 质量分数)的 Zn 和 Zn-In 合金在 6 M KOH 溶液中的腐蚀行为。塔菲尔图、电化学阻抗谱 (EIS)、循环伏安法 (CV) 和充放电方法都被采用。利用配备有色散能 X 射线 (EADX) 和 X 射线衍射的扫描电子显微镜 (SEM) 对 Zn 和 Zn-In 合金表面形成的腐蚀层的形态、化学成分和相进行了彻底研究。 (XRD), 分别。极化结果表明,Zn-1%In 合金的腐蚀保护效率在 45°C 时最高,为 97.3%。与锌相比,所研究合金的开路电位 (Ecorr.) 的稳态转移到更负的值。这表明锌与铟的合金化对充电效率、析氢反应的抑制和电容具有积极影响。此外,放电时间随着 Zn-In 双金属固溶体中铟百分比的增加而增加。合成的合金被认为是一种有前途的长寿命碱性电池材料。) 与锌相比,所研究的合金转移到一个更负的值。这表明锌与铟的合金化对充电效率、析氢反应的抑制和电容具有积极影响。此外,放电时间随着 Zn-In 双金属固溶体中铟百分比的增加而增加。合成的合金被认为是一种有前途的长寿命碱性电池材料。) 与锌相比,所研究的合金转移到一个更负的值。这表明锌与铟的合金化对充电效率、析氢反应的抑制和电容具有积极影响。此外,放电时间随着 Zn-In 双金属固溶体中铟百分比的增加而增加。合成的合金被认为是一种有前途的长寿命碱性电池材料。
更新日期:2021-02-01
中文翻译:

通过与铟合金化锌作为碱性电池阳极的电化学性能的发展
摘要 锌是碱性电池中主要使用的金属之一。在锌中添加微量铟作为合金元素可减缓腐蚀速度并促进锌的牺牲保护。电化学研究了不同 In 含量(0.5% 和 1%,In/Zn 质量分数)的 Zn 和 Zn-In 合金在 6 M KOH 溶液中的腐蚀行为。塔菲尔图、电化学阻抗谱 (EIS)、循环伏安法 (CV) 和充放电方法都被采用。利用配备有色散能 X 射线 (EADX) 和 X 射线衍射的扫描电子显微镜 (SEM) 对 Zn 和 Zn-In 合金表面形成的腐蚀层的形态、化学成分和相进行了彻底研究。 (XRD), 分别。极化结果表明,Zn-1%In 合金的腐蚀保护效率在 45°C 时最高,为 97.3%。与锌相比,所研究合金的开路电位 (Ecorr.) 的稳态转移到更负的值。这表明锌与铟的合金化对充电效率、析氢反应的抑制和电容具有积极影响。此外,放电时间随着 Zn-In 双金属固溶体中铟百分比的增加而增加。合成的合金被认为是一种有前途的长寿命碱性电池材料。) 与锌相比,所研究的合金转移到一个更负的值。这表明锌与铟的合金化对充电效率、析氢反应的抑制和电容具有积极影响。此外,放电时间随着 Zn-In 双金属固溶体中铟百分比的增加而增加。合成的合金被认为是一种有前途的长寿命碱性电池材料。) 与锌相比,所研究的合金转移到一个更负的值。这表明锌与铟的合金化对充电效率、析氢反应的抑制和电容具有积极影响。此外,放电时间随着 Zn-In 双金属固溶体中铟百分比的增加而增加。合成的合金被认为是一种有前途的长寿命碱性电池材料。


























 京公网安备 11010802027423号
京公网安备 11010802027423号