Insect Biochemistry and Molecular Biology ( IF 3.8 ) Pub Date : 2020-09-21 , DOI: 10.1016/j.ibmb.2020.103472 Jae-Hyuk Lee , Na-Rae Lee , Do-Hyoung Kim , Young-Joon Kim
|
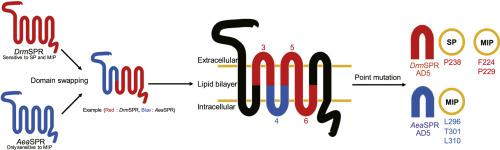
Drosophila melanogaster sex peptide receptor (DrmSPR) is a G protein-coupled receptor (GPCR) with ‘dual ligand selectivity’ towards sex peptide (SP) and myoinhibitory peptides (MIPs), which are only remotely related to one another. SPR is conserved in almost all the sequenced lophotrochozoan and ecdysozoan genomes. SPRs from non-drosophilid taxa, such as those from the mosquitoes Aedes aegypti (AeaSPR), Anopheles gambiae (AngSPR), and the sea slug Aplysia californica (ApcSPR), are highly sensitive to MIP, but not to SP. To understand how Drosophila SPRs evolved their SP sensitivity while maintaining MIP sensitivity, we examined ligand selectivity in a series of chimeric GPCRs that combine domains from the SP-sensitive DrmSPR and the SP-insensitive AeaSPR. We found replacement of Pro 238 (P238) in DrmSPR with the corresponding residue from AeaSPR (L310) reduced its SP sensitivity 2.7 fold without altering its MIP sensitivity. The P238 residue located in the third extracellular loop (ECL3) is conserved in Drosophila SPRs and in SPR from the moth Bombyx mori (BomSPR), which is considerably more sensitive to SP than AeaSPR, AngSPR, or ApcSPR. We found, however, that rather than improving AeaSPR's sensitivity to SP, replacement of L310 in AeaSPR with Pro significantly reduces its MIP sensitivity. Thus, our identification of a single amino acid residue critical for SP sensitivity, but not for MIP sensitivity is an important step in clarifying how DrmSPR evolved the ability to detect SP.
中文翻译:

果蝇和埃及伊蚊的性肽受体配体选择性的分子表征
果蝇性欲肽受体(Drmophila melanogaster)性肽受体(Drm SPR)是一种G蛋白偶联受体(GPCR),对性肽(SP)和肌抑制性肽(MIP)具有“双重配体选择性”,二者之间只有极短的关系。SPR在几乎所有测序的滋养体和蜕皮动物基因组中都是保守的。来自非果蝇类群的SPR对蚊子(AIP SPR)高度敏感,例如蚊子埃及伊蚊(Aea SPR),冈比亚按蚊(Ang SPR)和海参Aplysia californica(Apc SPR)的SPR。了解果蝇SPR在保持MIP敏感性的同时发展了SP敏感性,我们在一系列嵌合GPCR中检查了配体的选择性,这些GPCR结合了对SP敏感的Drm SPR和对SP不敏感的Aea SPR的结构域。我们发现用来自Aea SPR(L310)的相应残基替代Drm SPR中的Pro 238(P238),使其SP敏感性降低了2.7倍,而没有改变其MIP敏感性。位于果蝇SPR和蛾类家蚕(Bom SPR)的SPR中位于第三个细胞外环(ECL3)的P238残基是保守的,它比Aea SPR,Ang SPR或Apc对SP敏感得多SPR。但是,我们发现,用Pro替代Aea SPR中的L310并没有提高Aea SPR对SP的敏感性,而是大大降低了其MIP敏感性。因此,我们鉴定对SP敏感性至关重要但对MIP敏感性不重要的单个氨基酸残基是阐明Drm SPR如何演变检测SP能力的重要一步。


























 京公网安备 11010802027423号
京公网安备 11010802027423号