European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2020-09-21 , DOI: 10.1016/j.ejmech.2020.112863 Farooq-Ahmad Khan , Nourina Nasim , Yan Wang , Alaa Alhazmi , Mehar Sanam , Zaheer Ul-Haq , Damayanthi Yalamati , Marina Ulanova , Zi-Hua Jiang
|
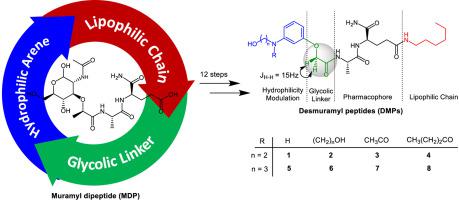
Nucleotide-binding oligomerization domain 2 (NOD2) is cytosolic surveillance receptor of the innate immune system capable of recognizing the bacterial and viral infections. Muramyl dipeptide (MDP) is the minimal immunoreactive unit of murein. NOD2 perceives MDP as pathogen-associated molecular pattern, thereby triggering an immune response with undesirable side-effects. Beneficial properties of MDP, such as pro-inflammatory characteristics for the rational design of new vaccine adjuvants, can be harnessed by strategically re-designing the molecule. In this work, a new class of amphiphilic desmuramylpeptides (DMPs) were synthesized by replacing the carbohydrate moiety (muramic acid) of the parent molecule with hydrophilic arenes. A lipophilic chain was also introduced at the C-terminus of dipeptide moiety (alanine-isoglutamine), while conserving its L-D configuration. These novel DMPs were found to set off the release of higher levels of tumour necrosis factor alpha (TNF-α) than Murabutide, which is a well-known NOD2 agonist. Molecular docking studies indicate that all these DMPs bind well to NOD2 receptor with similar dock scores (binding energy) through a number of hydrogen bonding and hydrophobic/π interactions with several crucial residues of the receptor. More studies are needed to further assess their immunomodulatory therapeutic potential, as well as the possible involvement of NOD2 activation.
中文翻译:

用于新型疫苗佐剂合理设计的两亲性去muramyl肽:合成,炎症反应的体外调节和分子对接研究
核苷酸结合寡聚结构域2(NOD2)是先天免疫系统的胞质监视受体,能够识别细菌和病毒感染。Muramyl二肽(MDP)是murein的最小免疫反应单位。NOD2将MDP视为与病原体相关的分子模式,从而触发具有不良副作用的免疫反应。MDP的有益特性,例如用于合理设计新疫苗佐剂的促炎特性,可以通过战略性地重新设计分子来加以利用。在这项工作中,通过用亲水性芳烃取代母体分子的碳水化合物部分(山mic酸),合成了一类新的两亲性去壁淀粉样肽(DMP)。亲脂性链也被引入二肽部分(丙氨酸-异谷氨酰胺)的C-末端,同时保留其LD配置。发现这些新颖的DMP引发了比众所周知的NOD2激动剂Murabutide高水平的肿瘤坏死因子α(TNF-α)释放。分子对接研究表明,所有这些DMP通过许多氢键和与受体几个关键残基的疏水/π相互作用,以相似的对接得分(结合能)与NOD2受体良好结合。需要更多的研究来进一步评估其免疫调节治疗潜力以及NOD2激活的可能参与。分子对接研究表明,所有这些DMP通过许多氢键和与受体几个关键残基的疏水/π相互作用,以相似的对接得分(结合能)与NOD2受体良好结合。需要更多的研究来进一步评估其免疫调节治疗潜力,以及可能涉及NOD2激活。分子对接研究表明,所有这些DMP通过许多氢键和与受体几个关键残基的疏水/π相互作用,以相似的对接得分(结合能)与NOD2受体良好结合。需要更多的研究来进一步评估其免疫调节治疗潜力,以及可能涉及NOD2激活。



























 京公网安备 11010802027423号
京公网安备 11010802027423号