当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Decarboxylative and Deaminative Alkylation of Difluoroenoxysilanes via Photoredox Catalysis: A General Method for Site-Selective Synthesis of Difluoroalkylated Alkanes.
Organic Letters ( IF 5.2 ) Pub Date : 2020-09-18 , DOI: 10.1021/acs.orglett.0c02997 Heng Song 1 , Ran Cheng 2 , Qiao-Qiao Min 2 , Xingang Zhang 1, 2
Organic Letters ( IF 5.2 ) Pub Date : 2020-09-18 , DOI: 10.1021/acs.orglett.0c02997 Heng Song 1 , Ran Cheng 2 , Qiao-Qiao Min 2 , Xingang Zhang 1, 2
Affiliation
|
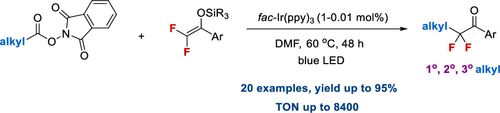
A general method for site-selective difluoroalkylation of alkyl carboxylic redox esters with difluoroenoxysilanes through photoredox-catalyzed decarboxylative reaction has been developed. The reaction can also be extended to aliphatic amine derived pyridinium salts. This method has the advantages of high efficiency, mild reaction conditions, and broad substrate scope, including primary, secondary, and sterically hindered tertiaryl alkyl substrates, providing a general and practical route for applications in organic synthesis and pharmaceutical studies.
中文翻译:

通过光氧化还原催化的二氟环氧乙烷的脱羧和脱氨基烷基化:选择性合成二氟烷基化烷烃的一般方法。
已经开发了通过光氧化还原催化的脱羧反应用二氟烯氧基硅烷对烷基羧酸氧化还原酯进行位点选择性二氟烷基化的一般方法。该反应还可以扩展至脂族胺衍生的吡啶鎓盐。该方法具有高效,温和的反应条件和广泛的底物范围的优点,包括伯,仲和空间受阻的叔芳基烷基底物,为有机合成和药物研究中的应用提供了通用和实用的途径。
更新日期:2020-10-02
中文翻译:

通过光氧化还原催化的二氟环氧乙烷的脱羧和脱氨基烷基化:选择性合成二氟烷基化烷烃的一般方法。
已经开发了通过光氧化还原催化的脱羧反应用二氟烯氧基硅烷对烷基羧酸氧化还原酯进行位点选择性二氟烷基化的一般方法。该反应还可以扩展至脂族胺衍生的吡啶鎓盐。该方法具有高效,温和的反应条件和广泛的底物范围的优点,包括伯,仲和空间受阻的叔芳基烷基底物,为有机合成和药物研究中的应用提供了通用和实用的途径。


























 京公网安备 11010802027423号
京公网安备 11010802027423号