当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chloroaluminate Liquid Clathrates: Is It the Cations or the Anions That Drive the Solubility of Aromatics?
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2020-09-18 , DOI: 10.1021/acs.iecr.0c03980 Rajkumar Kore 1 , Manish Kumar Mishra 1 , Uma Maheshwar Gonela 1 , Robin D. Rogers 1
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2020-09-18 , DOI: 10.1021/acs.iecr.0c03980 Rajkumar Kore 1 , Manish Kumar Mishra 1 , Uma Maheshwar Gonela 1 , Robin D. Rogers 1
Affiliation
|
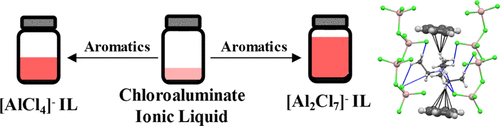
Three chloroaluminate ionic liquids (ILs), triethylammonium tetrachloroaluminate ([HN222][AlCl4]), triethylammonium heptachlorodialuminate ([HN222][Al2Cl7]), and tetraethylammonium heptachlorodialuminate ([N2222][Al2Cl7]), were employed to investigate liquid clathrate (LC) formation and the relative role of the cation or anion in aromatic solubility. The trend in molar solubilities in all three ILs was found to be benzene > toluene > o-xylene > p-xylene ∼ m-xylene > mesitylene; however, the solubilities in the [AlCl4]− IL were 40–70% lower than those found for the two [Al2Cl7]− ILs, which were nearly equivalent. Attempts to crystallize analogues of the LC phases from aromatic solutions of [N2222][Al2Cl7] led to crystalline [N2222][Al2Cl7]·C6H6 from benzene and [N2222][AlCl4] from all other aromatic solvents. NMR analyses of [HN222][AlCl4] and [HN222][Al2Cl7] LCs supported the crystallographic analyses, where 27Al NMR confirmed the dynamic speciation of the [Al2Cl7]− anions but not the [AlCl4]− anions, and 1H NMR supported the crystallographic observation that each benzene interacts with two cations; however, the solution data suggested a 2:1 benzene/IL ratio.
中文翻译:

氯铝酸盐的液体包合物:是阳离子还是阴离子驱动芳烃的溶解度?
三种氯铝酸盐离子液体(ILs),三乙铵四氯铝酸盐([HN 222 ] [AlCl 4 ]),三乙铵七氯二铝酸盐([HN 222 ] [Al 2 Cl 7 ])和四乙铵七氯二铝酸盐([N 2222 ] [Al 2 Cl 7 ] ),用于研究液体包合物(LC)的形成以及阳离子或阴离子在芳族溶解度中的相对作用。在所有三种离子液体的摩尔溶解度的趋势被发现是苯>甲苯> ö二甲苯> p二甲苯〜米二甲苯>均三甲苯; 但是,[AlCl 4 ]中的溶解度- IL分别为40-70%比找到的两个[铝下2氯7 ] -的离子液体,这是几乎相等。尝试从[N 2222 ] [Al 2 Cl 7 ]的芳族溶液中结晶出LC相的类似物,从而从苯和[N 2222 ] [AlCl 4 ]结晶为[N 2222 ] [Al 2 Cl 7 ]·C 6 H 6晶体]从所有其他芳香族溶剂中提取。[HN 222 ] [AlCl 4 ]和[HN 222 ] [Al 2 Cl 7] LC支持晶体学分析,其中27 Al NMR证实了[Al 2 Cl 7 ] -阴离子的动态形态,但没有[AlCl 4 ] -阴离子的动态形态,1 H NMR支持了晶体学观察,即每个苯与两个阳离子相互作用。 ; 但是,解决方案数据表明苯/ IL比为2:1。
更新日期:2020-10-15
中文翻译:

氯铝酸盐的液体包合物:是阳离子还是阴离子驱动芳烃的溶解度?
三种氯铝酸盐离子液体(ILs),三乙铵四氯铝酸盐([HN 222 ] [AlCl 4 ]),三乙铵七氯二铝酸盐([HN 222 ] [Al 2 Cl 7 ])和四乙铵七氯二铝酸盐([N 2222 ] [Al 2 Cl 7 ] ),用于研究液体包合物(LC)的形成以及阳离子或阴离子在芳族溶解度中的相对作用。在所有三种离子液体的摩尔溶解度的趋势被发现是苯>甲苯> ö二甲苯> p二甲苯〜米二甲苯>均三甲苯; 但是,[AlCl 4 ]中的溶解度- IL分别为40-70%比找到的两个[铝下2氯7 ] -的离子液体,这是几乎相等。尝试从[N 2222 ] [Al 2 Cl 7 ]的芳族溶液中结晶出LC相的类似物,从而从苯和[N 2222 ] [AlCl 4 ]结晶为[N 2222 ] [Al 2 Cl 7 ]·C 6 H 6晶体]从所有其他芳香族溶剂中提取。[HN 222 ] [AlCl 4 ]和[HN 222 ] [Al 2 Cl 7] LC支持晶体学分析,其中27 Al NMR证实了[Al 2 Cl 7 ] -阴离子的动态形态,但没有[AlCl 4 ] -阴离子的动态形态,1 H NMR支持了晶体学观察,即每个苯与两个阳离子相互作用。 ; 但是,解决方案数据表明苯/ IL比为2:1。

























 京公网安备 11010802027423号
京公网安备 11010802027423号