当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Defluorinative Ring‐Opening Indolylation of Siloxydifluorocyclopropanes: Controlled Synthesis of α‐Fluoro‐β‐Indolyl‐Propanones for Carbazole Construction
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-09-17 , DOI: 10.1002/adsc.202000865 Xiaowei Liu 1 , Dongxu Du 1 , Shuting Li 1 , Xin Wang 2 , Cong Xu 1 , Mang Wang 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-09-17 , DOI: 10.1002/adsc.202000865 Xiaowei Liu 1 , Dongxu Du 1 , Shuting Li 1 , Xin Wang 2 , Cong Xu 1 , Mang Wang 1
Affiliation
|
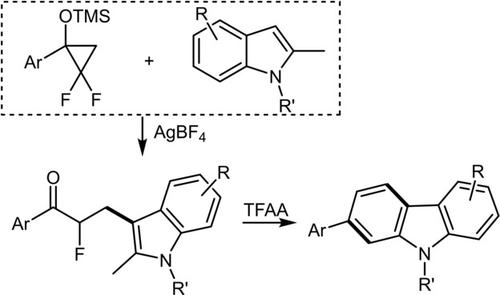
A catalytic defluorinative ring‐opening indolylation of siloxydifluorocyclopropanes was reported. It was found that AgBF4‐catalyzed reaction of siloxydifluorocyclopropanes with indoles could be controlled to deliver α‐fluoro‐β‐indolyl‐propanones within 1.5 hours at room temperature. Cyclization of these α‐fluoroketone derivatives were then carried out in the presence of trifluoroacetic anhydride in toluene at room temperature. Carbazoles were formed efficiently via an intramolecularly nucleophilic addition of the in situ formed enamine intermediate to the keto carbonyl, followed by a sequential hydrolysis and eliminations of trifluoroacetic acid and hydrogen fluoride.
中文翻译:

甲硅烷氧基二氟环丙烷的脱氟开环吲哚化:用于咔唑构建的α-氟-β-吲哚基-丙烷的受控合成
据报道,甲硅烷氧基二氟环丙烷具有催化脱氟开环吲哚基。发现在室温下,可以控制AgBF 4催化的硅烷氧基二氟环丙烷与吲哚的反应,以在1.5小时内释放α-氟-β-吲哚基-丙烷。然后在室温下在甲苯中三氟乙酸酐存在下对这些α-氟代酮衍生物进行环化。通过分子内亲核将原位形成的烯胺中间体加到酮羰基上,可有效地形成咔唑,然后依次水解并消除三氟乙酸和氟化氢。
更新日期:2020-11-19
中文翻译:

甲硅烷氧基二氟环丙烷的脱氟开环吲哚化:用于咔唑构建的α-氟-β-吲哚基-丙烷的受控合成
据报道,甲硅烷氧基二氟环丙烷具有催化脱氟开环吲哚基。发现在室温下,可以控制AgBF 4催化的硅烷氧基二氟环丙烷与吲哚的反应,以在1.5小时内释放α-氟-β-吲哚基-丙烷。然后在室温下在甲苯中三氟乙酸酐存在下对这些α-氟代酮衍生物进行环化。通过分子内亲核将原位形成的烯胺中间体加到酮羰基上,可有效地形成咔唑,然后依次水解并消除三氟乙酸和氟化氢。



























 京公网安备 11010802027423号
京公网安备 11010802027423号