当前位置:
X-MOL 学术
›
Drug Test. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Elimination profiles of betamethasone after different administration routes: Evaluation of the reporting level and washout periods to ensure safe therapeutic administrations
Drug Testing and Analysis ( IF 2.9 ) Pub Date : 2020-09-18 , DOI: 10.1002/dta.2928 Sergi Coll 1 , Núria Monfort 1 , Élida Alechaga 1 , Xavier Matabosch 1 , Clara Pérez-Mañá 2, 3 , Rosa Ventura 1, 4
Drug Testing and Analysis ( IF 2.9 ) Pub Date : 2020-09-18 , DOI: 10.1002/dta.2928 Sergi Coll 1 , Núria Monfort 1 , Élida Alechaga 1 , Xavier Matabosch 1 , Clara Pérez-Mañá 2, 3 , Rosa Ventura 1, 4
Affiliation
|
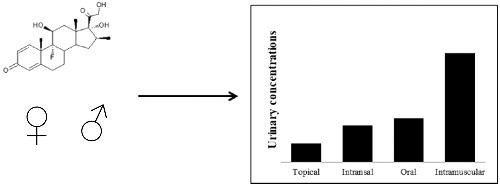
Betamethasone (BET) is prohibited in sports competitions when administered by systemic routes, and it is allowed by other routes for therapeutic purposes. In out‐of‐competition periods, there is no restriction of use. The present work aimed to assess the urinary excretion of BET and its metabolites after allowed and prohibited administrations to verify the suitability of the current reporting level of 30 ng/ml used to distinguish allowed and prohibited administrations and to establish washout periods for oral and intramuscular (IM) administrations when out‐of‐competition treatments are needed. BET was administered to healthy volunteers by different routes: topical (10 mg/day for 5 days, n = 6 males), intranasal (320 μg/day for 3 days, n = 4 males and 4 females), oral (0.5 mg, n = 8 males) or IM (6 mg, n = 6 males, or 12 mg, n = 4 males and 4 females). Urine and plasma samples collected before and after administration were analysed using liquid chromatography–tandem mass spectrometry. Among all studied metabolites, the parent drug was selected as the best discriminatory marker. After topical administration, BET concentrations were lower than 6.6 ng/ml. However, after intranasal treatment, some samples at concentrations close to or higher than 30 ng/ml were detected, suggesting the need to revise the current reporting level. Urinary concentrations after oral and intranasal administrations were similar, and after IM administration, concentrations were much higher. Taking into account all information, a urinary reporting level of 60 ng/ml is proposed. Washout periods of at least 48 and 96 h are recommended after oral and IM administrations, respectively.
中文翻译:

不同给药途径后倍他米松的消除曲线:评估报告水平和清除期以确保安全的治疗给药
体育比赛中禁止通过全身给药途径使用倍他米松 (BET),但出于治疗目的允许通过其他途径给药。在赛外期间,没有使用限制。目前的工作旨在评估允许和禁止给药后 BET 及其代谢物的尿排泄,以验证当前报告水平 30 ng/ml 的适用性,用于区分允许和禁止给药,并确定口服和肌肉注射的洗脱期。 IM) 需要赛外治疗时的给药。BET 通过不同途径给予健康志愿者:局部(10 毫克/天,连续 5 天,n = 6 名男性)、鼻内(320 微克/天,连续 3 天,n = 4 名男性和 4 名女性)、口服(0.5 毫克,n = 8 男性)或 IM(6 毫克,n = 6 男性,或 12 毫克,n= 4 男 4 女)。使用液相色谱-串联质谱法分析给药前后收集的尿液和血浆样品。在所有研究的代谢物中,母体药物被选为最好的区分标记。局部给药后,BET 浓度低于 6.6 ng/ml。然而,在鼻内治疗后,一些样品的浓度接近或高于 30 ng/ml,表明需要修改当前的报告水平。口服和鼻内给药后的尿液浓度相似,而肌内给药后,浓度要高得多。考虑到所有信息,建议尿液报告水平为 60 ng/ml。分别推荐口服和肌注给药后至少 48 小时和 96 小时的洗脱期。
更新日期:2020-09-18
中文翻译:

不同给药途径后倍他米松的消除曲线:评估报告水平和清除期以确保安全的治疗给药
体育比赛中禁止通过全身给药途径使用倍他米松 (BET),但出于治疗目的允许通过其他途径给药。在赛外期间,没有使用限制。目前的工作旨在评估允许和禁止给药后 BET 及其代谢物的尿排泄,以验证当前报告水平 30 ng/ml 的适用性,用于区分允许和禁止给药,并确定口服和肌肉注射的洗脱期。 IM) 需要赛外治疗时的给药。BET 通过不同途径给予健康志愿者:局部(10 毫克/天,连续 5 天,n = 6 名男性)、鼻内(320 微克/天,连续 3 天,n = 4 名男性和 4 名女性)、口服(0.5 毫克,n = 8 男性)或 IM(6 毫克,n = 6 男性,或 12 毫克,n= 4 男 4 女)。使用液相色谱-串联质谱法分析给药前后收集的尿液和血浆样品。在所有研究的代谢物中,母体药物被选为最好的区分标记。局部给药后,BET 浓度低于 6.6 ng/ml。然而,在鼻内治疗后,一些样品的浓度接近或高于 30 ng/ml,表明需要修改当前的报告水平。口服和鼻内给药后的尿液浓度相似,而肌内给药后,浓度要高得多。考虑到所有信息,建议尿液报告水平为 60 ng/ml。分别推荐口服和肌注给药后至少 48 小时和 96 小时的洗脱期。



























 京公网安备 11010802027423号
京公网安备 11010802027423号