当前位置:
X-MOL 学术
›
Luminescence
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Bovine β‐casein binding studies of a Schiff base ligand: fluorescence and circular dichroism approaches
Luminescence ( IF 2.9 ) Pub Date : 2020-09-17 , DOI: 10.1002/bio.3951 Nooshin Sarreshtehdari 1 , Fatemeh S Mohseni-Shahri 1 , Farid Moeinpour 1
Luminescence ( IF 2.9 ) Pub Date : 2020-09-17 , DOI: 10.1002/bio.3951 Nooshin Sarreshtehdari 1 , Fatemeh S Mohseni-Shahri 1 , Farid Moeinpour 1
Affiliation
|
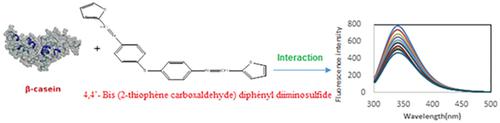
In this study, a Schiff base derived from a heterocyclic moiety was synthesized and characterized. The in vitro binding behaviour of this ligand with β‐casein (β‐CN) was investigated using biophysical techniques. For evaluation, thermodynamics variables of interactions between the Schiff base ligand and β‐CN, such as fluorescence at different temperatures, were measured. The results showed that the Schiff base ligand possessed considerable associated binding to β‐CN and that the procedure was enthalpy driven. The β‐CN conformation was also changed to give a further unfolded structure. Fluorescence resonance energy transfer was used to estimate the interval between donor (β‐CN) and acceptor (Schiff base ligand). All these experimental results proposed that β‐CN might act as carrier protein for the Schiff base ligand to deliver it to the target molecules.
中文翻译:

牛β-酪蛋白结合席夫碱配体的研究:荧光和圆二色性方法
在这项研究中,合成并表征了衍生自杂环部分的席夫碱。在体外使用生物物理技术研究了该配体与β-酪蛋白(β-CN)的结合行为。为了进行评估,测量了席夫碱配体与β-CN之间相互作用的热力学变量,例如在不同温度下的荧光。结果表明,席夫碱配体与β-CN具有相当大的缔合结合,并且该过程是焓驱动的。β-CN构象也被改变以提供进一步的未折叠结构。荧光共振能量转移用于估计供体(β-CN)与受体(席夫碱配体)之间的间隔。所有这些实验结果表明,β-CN可能充当席夫碱配体的载体蛋白,将其传递至靶分子。
更新日期:2020-09-17
中文翻译:

牛β-酪蛋白结合席夫碱配体的研究:荧光和圆二色性方法
在这项研究中,合成并表征了衍生自杂环部分的席夫碱。在体外使用生物物理技术研究了该配体与β-酪蛋白(β-CN)的结合行为。为了进行评估,测量了席夫碱配体与β-CN之间相互作用的热力学变量,例如在不同温度下的荧光。结果表明,席夫碱配体与β-CN具有相当大的缔合结合,并且该过程是焓驱动的。β-CN构象也被改变以提供进一步的未折叠结构。荧光共振能量转移用于估计供体(β-CN)与受体(席夫碱配体)之间的间隔。所有这些实验结果表明,β-CN可能充当席夫碱配体的载体蛋白,将其传递至靶分子。



























 京公网安备 11010802027423号
京公网安备 11010802027423号