当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Colorectal Tumor Microenvironment-Activated Bio-Decomposable and Metabolizable Cu2 O@CaCO3 Nanocomposites for Synergistic Oncotherapy.
Advanced Materials ( IF 29.4 ) Pub Date : 2020-09-18 , DOI: 10.1002/adma.202004647 Mengyu Chang 1, 2 , Zhiyao Hou 1, 3, 4 , Dayong Jin 5, 6 , Jiajia Zhou 5 , Man Wang 7 , Meifang Wang 1, 2 , Mengmeng Shu 1 , Binbin Ding 1, 2 , Chunxia Li 7 , Jun Lin 1, 2
Advanced Materials ( IF 29.4 ) Pub Date : 2020-09-18 , DOI: 10.1002/adma.202004647 Mengyu Chang 1, 2 , Zhiyao Hou 1, 3, 4 , Dayong Jin 5, 6 , Jiajia Zhou 5 , Man Wang 7 , Meifang Wang 1, 2 , Mengmeng Shu 1 , Binbin Ding 1, 2 , Chunxia Li 7 , Jun Lin 1, 2
Affiliation
|
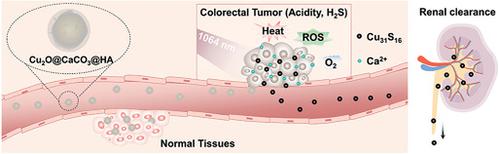
Rational design of tumor microenvironment (TME)‐activated nanocomposites provides an innovative strategy to construct responsive oncotherapy. In colorectal cancer (CRC), the specific physiological features are the overexpressed endogenous H2S and slightly acidic microenvironment. Here, a core–shell Cu2O@CaCO3 nanostructure for CRC “turn‐on” therapy is reported. With CaCO3 responsive to pH decomposition and Cu2O responsive to H2S sulfuration, Cu2O@CaCO3 can be triggered “on” into the therapeutic mode by the colorectal TME. When the CaCO3 shell decomposes and releases calcium in acidic colorectal TME, the loss of protection from the CaCO3 shell exposes the Cu2O core to be sulfuretted by H2S to form metabolizable Cu31S16 nanocrystals that gain remarkably strong near‐infrared absorption. After modifying hyaluronic acid, Cu2O@CaCO3 can achieve synergistic CRC‐targeted and TME‐triggered photothermal/photodynamic/chemodynamic/calcium‐overload‐mediated therapy. Moreover, it is found that the generation of hyperthermia and oxidative stress from Cu2O@CaCO3 nanocomposites can efficiently reprogram the macrophages from the M2 phenotype to the M1 phenotype and initiate a vaccine‐like immune effect after primary tumor removal, which further induces an immune‐favorable TME and intense immune responses for anti‐CD47 antibody to simultaneously inhibit CRC distant metastasis and recurrence by immunotherapy.
中文翻译:

大肠肿瘤微环境激活的生物可分解和可代谢的Cu2O @ CaCO3纳米复合材料,用于协同肿瘤治疗。
肿瘤微环境(TME)活化的纳米复合材料的合理设计为构建响应性肿瘤疗法提供了创新的策略。在结直肠癌(CRC)中,特定的生理特征是过表达的内源性H 2 S和微酸性的微环境。此处报道了用于CRC“开启”治疗的核壳型Cu 2 O @ CaCO 3纳米结构。由于CaCO 3对pH分解有响应,而Cu 2 O对H 2 S硫化有响应,因此Cu 2 O @ CaCO 3可以通过结直肠TME触发“进入”治疗模式。当CaCO 3壳在酸性结直肠TME中分解并释放钙,CaCO 3壳的保护作用丧失,使Cu 2 O核暴露于H 2 S的硫化作用下,形成可代谢的Cu 31 S 16纳米晶体,从而获得明显的近红外吸收。修饰透明质酸后,Cu 2 O @ CaCO 3可以实现协同的CRC靶向和TME触发的光热/光动力/化学动力/钙超载介导的治疗。此外,发现由Cu 2 O @ CaCO 3产生高温和氧化应激 纳米复合物可以有效地将巨噬细胞从M2表型重编程为M1表型,并在原发肿瘤切除后启动疫苗样的免疫作用,从而进一步诱导针对CD47抗体的免疫有利的TME和强烈的免疫反应,同时抑制CRC远处转移并通过免疫疗法复发。
更新日期:2020-10-26
中文翻译:

大肠肿瘤微环境激活的生物可分解和可代谢的Cu2O @ CaCO3纳米复合材料,用于协同肿瘤治疗。
肿瘤微环境(TME)活化的纳米复合材料的合理设计为构建响应性肿瘤疗法提供了创新的策略。在结直肠癌(CRC)中,特定的生理特征是过表达的内源性H 2 S和微酸性的微环境。此处报道了用于CRC“开启”治疗的核壳型Cu 2 O @ CaCO 3纳米结构。由于CaCO 3对pH分解有响应,而Cu 2 O对H 2 S硫化有响应,因此Cu 2 O @ CaCO 3可以通过结直肠TME触发“进入”治疗模式。当CaCO 3壳在酸性结直肠TME中分解并释放钙,CaCO 3壳的保护作用丧失,使Cu 2 O核暴露于H 2 S的硫化作用下,形成可代谢的Cu 31 S 16纳米晶体,从而获得明显的近红外吸收。修饰透明质酸后,Cu 2 O @ CaCO 3可以实现协同的CRC靶向和TME触发的光热/光动力/化学动力/钙超载介导的治疗。此外,发现由Cu 2 O @ CaCO 3产生高温和氧化应激 纳米复合物可以有效地将巨噬细胞从M2表型重编程为M1表型,并在原发肿瘤切除后启动疫苗样的免疫作用,从而进一步诱导针对CD47抗体的免疫有利的TME和强烈的免疫反应,同时抑制CRC远处转移并通过免疫疗法复发。



























 京公网安备 11010802027423号
京公网安备 11010802027423号