当前位置:
X-MOL 学术
›
J. Electroanal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effect of aggregation on the simple ion transfer across oil water interfaces
Journal of Electroanalytical Chemistry ( IF 4.5 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.jelechem.2020.114678 F.M. Zanotto , R.A. Fernández , S.A. Dassie
Journal of Electroanalytical Chemistry ( IF 4.5 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.jelechem.2020.114678 F.M. Zanotto , R.A. Fernández , S.A. Dassie
|
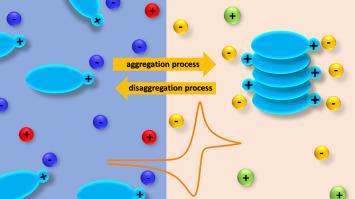
Abstract The theory of cyclic voltammetry of simple ion transfer across a liquid liquid (L L) interface including the effect of aggregation is developed. We focus on two different situations: ion transfer followed by an aggregation process in the organic phase, and a disaggregation process in the aqueous phase followed by ion transfer. We use parameters typical of the areas of study of molecular aggregation and polymer formation, namely average aggregate size and polydispersity index. These parameters allow us to analyse, in a simple and rigorous way, the interconversion between monomer and aggregate species, both at the aqueous or organic side of the L L interface and in the interfacial region during the potential scan. Our results show that aggregation occurring in the organic phase facilitates ion transfer across the L L interface. In contrast, when the aggregates are present in the aqueous phase, they must be disaggregated so that the monomers can be transferred, hence the transfer energy increases. Both processes significantly alter the shape of voltammograms. The peak current and mid-peak potential are analysed as a function of initial concentrations, aggregation constants and maximum number of monomers forming each aggregate. These results are compared with approximate analytical equations, which can be used to guide further experiments and extract information from experimental responses.
中文翻译:

聚集对跨油水界面的简单离子转移的影响
摘要 发展了包括聚集效应在内的简单离子穿过液液 (LL) 界面的循环伏安法理论。我们关注两种不同的情况:离子转移后有机相中的聚集过程,以及水相中的解聚过程后离子转移。我们使用分子聚集和聚合物形成研究领域的典型参数,即平均聚集体尺寸和多分散指数。这些参数使我们能够以简单而严格的方式分析单体和聚集体物种之间的相互转化,无论是在 LL 界面的水侧或有机侧,还是在电位扫描期间的界面区域。我们的结果表明,有机相中发生的聚集促进了离子穿过 LL 界面的转移。相比之下,当聚集体存在于水相中时,它们必须被解聚以便单体可以被转移,因此转移能增加。这两个过程都显着改变了伏安图的形状。峰值电流和中间峰值电位作为初始浓度、聚集常数和形成每个聚集体的最大单体数量的函数进行分析。将这些结果与近似解析方程进行比较,可用于指导进一步的实验并从实验响应中提取信息。峰值电流和中间峰值电位作为初始浓度、聚集常数和形成每个聚集体的最大单体数量的函数进行分析。将这些结果与近似解析方程进行比较,可用于指导进一步的实验并从实验响应中提取信息。峰值电流和中间峰值电位作为初始浓度、聚集常数和形成每个聚集体的最大单体数量的函数进行分析。将这些结果与近似解析方程进行比较,可用于指导进一步的实验并从实验响应中提取信息。
更新日期:2020-12-01
中文翻译:

聚集对跨油水界面的简单离子转移的影响
摘要 发展了包括聚集效应在内的简单离子穿过液液 (LL) 界面的循环伏安法理论。我们关注两种不同的情况:离子转移后有机相中的聚集过程,以及水相中的解聚过程后离子转移。我们使用分子聚集和聚合物形成研究领域的典型参数,即平均聚集体尺寸和多分散指数。这些参数使我们能够以简单而严格的方式分析单体和聚集体物种之间的相互转化,无论是在 LL 界面的水侧或有机侧,还是在电位扫描期间的界面区域。我们的结果表明,有机相中发生的聚集促进了离子穿过 LL 界面的转移。相比之下,当聚集体存在于水相中时,它们必须被解聚以便单体可以被转移,因此转移能增加。这两个过程都显着改变了伏安图的形状。峰值电流和中间峰值电位作为初始浓度、聚集常数和形成每个聚集体的最大单体数量的函数进行分析。将这些结果与近似解析方程进行比较,可用于指导进一步的实验并从实验响应中提取信息。峰值电流和中间峰值电位作为初始浓度、聚集常数和形成每个聚集体的最大单体数量的函数进行分析。将这些结果与近似解析方程进行比较,可用于指导进一步的实验并从实验响应中提取信息。峰值电流和中间峰值电位作为初始浓度、聚集常数和形成每个聚集体的最大单体数量的函数进行分析。将这些结果与近似解析方程进行比较,可用于指导进一步的实验并从实验响应中提取信息。


























 京公网安备 11010802027423号
京公网安备 11010802027423号