Computational and Structural Biotechnology Journal ( IF 6 ) Pub Date : 2020-09-20 , DOI: 10.1016/j.csbj.2020.09.017 Ekaterina Shevchenko , Antti Poso , Tatu Pantsar
|
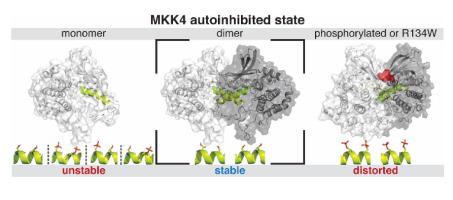
Protein kinases are crucial components of the cell-signalling machinery that orchestrate and convey messages to their downstream targets. Most often, kinases are activated upon a phosphorylation to their activation loop, which will shift the kinase into the active conformation. The Dual specificity mitogen-activated protein kinase kinase 4 (MKK4) exists in a unique conformation in its inactive unphosphorylated state, where its activation segment appears in a stable α-helical conformation. However, the precise role of this unique conformational state of MKK4 is unknown. Here, by all-atom molecular dynamics simulations (MD simulations), we show that this inactive state is unstable as monomer even when unphosphorylated and that the phosphorylation of the activation segment further destabilizes the autoinhibited α-helix. The specific phosphorylation pattern of the activation segment has also a unique influence on MKK4 dynamics. Furthermore, we observed that this specific inactive state is stable as a dimer, which becomes destabilized upon phosphorylation. Finally, we noticed that the most frequent MKK4 mutation observed in cancer, R134W, which role has not been disclosed to date, contributes to the dimer stability. Based on these data we postulate that MKK4 occurs as a dimer in its inactive autoinhibited state, providing an additional layer for its activity regulation.
中文翻译:

MKK4的自动抑制状态:磷酸化,推定二聚和R134W突变体的分子动力学模拟研究。
蛋白激酶是细胞信号传递机制的重要组成部分,可以协调并向下游目标传递信息。最常见的是,激酶被磷酸化至其激活环后被激活,这会将激酶转变为活性构象。双特异性有丝分裂原激活的蛋白激酶激酶4(MKK4)以其非活性的非磷酸化状态以独特的构象存在,其中其激活片段以稳定的α-螺旋构象出现。但是,未知的MKK4这种独特的构象状态的确切作用。在这里,通过全原子分子动力学模拟(MD模拟),我们表明,即使未磷酸化,这种非活性状态作为单体也是不稳定的,并且活化链段的磷酸化进一步破坏了自抑制的α-螺旋。激活片段的特定磷酸化模式对MKK4动力学也有独特的影响。此外,我们观察到该特定的非活性状态作为二聚体是稳定的,其在磷酸化时变得不稳定。最后,我们注意到在癌症中观察到的最常见的MKK4突变R134W迄今尚未公开其作用,有助于形成二聚体。基于这些数据,我们推测MKK4以二聚体形式出现在其非活性的自抑制状态,为它的活性调节提供了额外的一层。迄今为止尚未公开哪个作用有助于二聚体的稳定性。基于这些数据,我们推测MKK4以二聚体形式出现在其非活性的自抑制状态,从而为其活性调节提供了额外的一层。迄今尚未公开哪个作用有助于二聚体的稳定性。基于这些数据,我们推测MKK4以二聚体形式出现在其非活性的自抑制状态,从而为其活性调节提供了额外的一层。

























 京公网安备 11010802027423号
京公网安备 11010802027423号