Computational and Structural Biotechnology Journal ( IF 6 ) Pub Date : 2020-09-18 , DOI: 10.1016/j.csbj.2020.09.019 Ratul Chowdhury 1 , Veda Sheersh Boorla 1 , Costas D Maranas 1
|
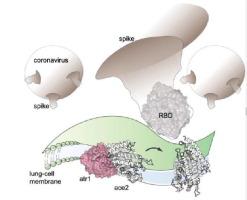
SARS-CoV-2 is a novel highly virulent pathogen which gains entry to human cells by binding with the cell surface receptor – angiotensin converting enzyme (ACE2). We computationally contrasted the binding interactions between human ACE2 and coronavirus spike protein receptor binding domain (RBD) of the 2002 epidemic-causing SARS-CoV-1, SARS-CoV-2, and bat coronavirus RaTG13 using the Rosetta energy function. We find that the RBD of the spike protein of SARS-CoV-2 is highly optimized to achieve very strong binding with human ACE2 (hACE2) which is consistent with its enhanced infectivity. SARS-CoV-2 forms the most stable complex with hACE2 compared to SARS-CoV-1 (23% less stable) or RaTG13 (11% less stable). Notably, we calculate that the SARS-CoV-2 RBD lowers the binding strength of angiotensin 2 receptor type I (ATR1) which is the native binding partner of ACE2 by 44.2%. Strong binding is mediated through strong electrostatic attachments with every fourth residue on the N-terminus alpha-helix (starting from Ser19 to Asn53) as the turn of the helix makes these residues solvent accessible. By contrasting the spike protein SARS-CoV-2 Rosetta binding energy with ACE2 of different livestock and pet species we find strongest binding with bat ACE2 followed by human, feline, equine, canine and finally chicken. This is consistent with the hypothesis that bats are the viral origin and reservoir species. These results offer a computational explanation for the increased infection susceptibility by SARS-CoV-2 and allude to therapeutic modalities by identifying and rank-ordering the ACE2 residues involved in binding with the virus.
中文翻译:

SARS-CoV-2 刺突蛋白与 ACE2 受体结合的计算生物物理特征及其对感染性的影响
SARS-CoV-2 是一种新型高毒病原体,通过与细胞表面受体——血管紧张素转换酶(ACE2)结合进入人体细胞。我们使用 Rosetta 能量函数计算对比了人类 ACE2 与 2002 年引起流行的 SARS-CoV-1、SARS-CoV-2 和蝙蝠冠状病毒 RaTG13 的冠状病毒刺突蛋白受体结合域 (RBD) 之间的结合相互作用。我们发现 SARS-CoV-2 刺突蛋白的 RBD 经过高度优化,可与人 ACE2 (hACE2) 实现非常强的结合,这与其增强的感染性相一致。与 SARS-CoV-1(稳定性降低 23%)或 RaTG13(稳定性降低 11%)相比,SARS-CoV-2 与 hACE2 形成最稳定的复合物。值得注意的是,我们计算出 SARS-CoV-2 RBD 使 ACE2 天然结合伴侣 I 型血管紧张素 2 受体 (ATR1) 的结合强度降低了 44.2%。强结合是通过 N 末端 α 螺旋(从 Ser19 到 Asn53 开始)上每四个残基的强静电附着介导的,因为螺旋的转动使这些残基可被溶剂接触。通过比较刺突蛋白 SARS-CoV-2 Rosetta 与不同牲畜和宠物物种的 ACE2 的结合能,我们发现与蝙蝠 ACE2 的结合最强,其次是人类、猫科动物、马科动物、犬科动物,最后是鸡。这与蝙蝠是病毒起源和储存物种的假设是一致的。这些结果为 SARS-CoV-2 感染易感性增加提供了计算解释,并通过识别与病毒结合相关的 ACE2 残基并对其进行排序来暗示治疗方式。



























 京公网安备 11010802027423号
京公网安备 11010802027423号