Acta Pharmaceutica Sinica B ( IF 14.5 ) Pub Date : 2020-09-19 , DOI: 10.1016/j.apsb.2020.09.007 Yifan Chen , Xuejing Shao , Ji Cao , Hong Zhu , Bo Yang , Qiaojun He , Meidan Ying
|
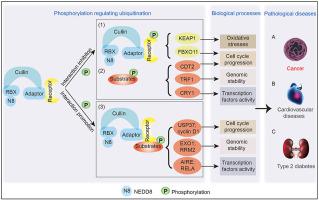
Cullin-RING ligases (CRLs) recognize and interact with substrates for ubiquitination and degradation, and can be targeted for disease treatment when the abnormal expression of substrates involves pathologic processes. Phosphorylation, either of substrates or receptors of CRLs, can alter their interaction. Phosphorylation-dependent ubiquitination and proteasome degradation influence various cellular processes and can contribute to the occurrence of various diseases, most often tumorigenesis. These processes have the potential to be used for tumor intervention through the regulation of the activities of related kinases, along with the regulation of the stability of specific oncoproteins and tumor suppressors. This review describes the mechanisms and biological functions of crosstalk between phosphorylation and ubiquitination, and most importantly its influence on tumorigenesis, to provide new directions and strategies for tumor therapy.
中文翻译:

磷酸化调节肿瘤发生中基于cullin的泛素化
Cullin-ring连接酶(CRL)识别底物并与底物相互作用,以进行泛素化和降解,当底物的异常表达涉及病理过程时,可作为疾病治疗的靶标。CRLs的底物或受体的磷酸化可改变其相互作用。磷酸化依赖性泛素化和蛋白酶体降解影响各种细胞过程,并可能导致多种疾病的发生,最常见的是肿瘤发生。这些过程通过调节相关激酶的活性,以及调节特定癌蛋白和肿瘤抑制物的稳定性,具有用于肿瘤干预的潜力。这篇综述描述了磷酸化和泛素化之间的串扰的机制和生物学功能,



























 京公网安备 11010802027423号
京公网安备 11010802027423号