当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Copper(I)-catalyzed asymmetric [3 + 3] annulation involving aziridines to construct tetrahydro-β-carbolines
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-09-17 , DOI: 10.1039/d0qo00742k Chao Ye 1, 2, 3, 4, 5 , Wu-Lin Yang 1, 5, 6, 7, 8 , Yufei Zhai 5, 6, 7, 8 , Hua Deng 1, 2, 3, 4, 5 , Xiaoyan Luo 1, 2, 3, 4, 5 , Guoyin Kai 5, 6, 7, 8 , Wei-Ping Deng 1, 5, 6, 7, 8
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-09-17 , DOI: 10.1039/d0qo00742k Chao Ye 1, 2, 3, 4, 5 , Wu-Lin Yang 1, 5, 6, 7, 8 , Yufei Zhai 5, 6, 7, 8 , Hua Deng 1, 2, 3, 4, 5 , Xiaoyan Luo 1, 2, 3, 4, 5 , Guoyin Kai 5, 6, 7, 8 , Wei-Ping Deng 1, 5, 6, 7, 8
Affiliation
|
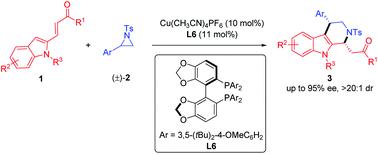
A catalytic asymmetric [3 + 3] annulation of aziridines with substituted 2-vinylindoles was developed. The reaction proceeds through copper-catalyzed ring-opening of aziridines followed by base-promoted intramolecular aza-Michael addition in a one-pot process. This strategy provides an efficient method for the direct synthesis of 1,4-disubstituted tetrahydro-β-carbolines with generally high diastereo- and enantioselectivities (up to >20 : 1 dr, 96% ee).
中文翻译:

铜(I)催化的涉及氮丙啶的不对称[3 + 3]环化反应,以构建四氢-β-咔啉
开发了具有取代的2-乙烯基吲哚的氮丙啶催化不对称[3 + 3]环。该反应通过铜催化的氮丙啶的开环进行,然后通过一锅法进行碱促进的分子内氮杂-Michael加成反应。该策略提供了一种直接合成具有高非对映选择性和对映选择性(最高> 20:1 dr,96%ee)的1,4-二取代的四氢-β-咔啉的有效方法。
更新日期:2020-09-25
中文翻译:

铜(I)催化的涉及氮丙啶的不对称[3 + 3]环化反应,以构建四氢-β-咔啉
开发了具有取代的2-乙烯基吲哚的氮丙啶催化不对称[3 + 3]环。该反应通过铜催化的氮丙啶的开环进行,然后通过一锅法进行碱促进的分子内氮杂-Michael加成反应。该策略提供了一种直接合成具有高非对映选择性和对映选择性(最高> 20:1 dr,96%ee)的1,4-二取代的四氢-β-咔啉的有效方法。


























 京公网安备 11010802027423号
京公网安备 11010802027423号