当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Practical Synthesis of [(tmeda)Ni(CH3)2], Isotopically Labeled [(tmeda)Ni(13CH3)2], and Neutral Chelated-Nickel Methyl Complexes
Organometallics ( IF 2.8 ) Pub Date : 2020-09-17 , DOI: 10.1021/acs.organomet.0c00500 Inigo Göttker-Schnetmann 1 , Stefan Mecking 1
Organometallics ( IF 2.8 ) Pub Date : 2020-09-17 , DOI: 10.1021/acs.organomet.0c00500 Inigo Göttker-Schnetmann 1 , Stefan Mecking 1
Affiliation
|
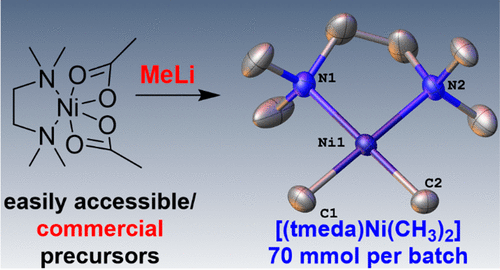
A practical procedure for the synthesis of N,N,N′,N′-tetramethylethylene diamine nickel dimethyl, [(tmeda)Ni(CH3)2] (5), based on commercially available methyllithium and [(tmeda)Ni(acac)2] (1) (acac = acetylacetonate), [(tmeda)Ni(OAc)2] (2) (OAc = acetate), [(tmeda)NiSO4] (3), or [(tmeda)NiF2] (4) has been developed. With 2 as the starting material, reproducible isolation of >14 g (ca. 68 mmol, 85%) of 5 in one-batch reactions is feasible. This synthetic route also enables the formation and isolation of 13C-labeled [(tmeda)Ni(13CH3)2] (5-13CH3) starting from 13CH3I by intermediate isolation of [(tmeda)2–3(Li13CH3)4]x (10). The amount of iodide impurities in 10 to push back iodide-catalyzed decomposition of 5-13CH3 to ethane and nickel black by a Kumada-type coupling is essential for successful isolation of 5-13CH3. [(tmeda)Ni(OAc)2] (2) is a superior nickel precursor to 5 in comparison to [(tmeda)Ni(acac)2] (1), as (a) removal of lithium acetate (9) from the crude reaction mixture is much easier than removal of lithium acetylacetonate and (b) κ2-N,O-salicylaldiminato nickel methyl complexes (12) or a κ2-P,O-phosphinesulfonato nickel methyl complex (14) can be easily prepared in a one-pot procedure starting from 2, methyllithium, and salicylaldimines (11) or phosphoniumsulfonate (13), respectively. The X-ray diffraction analyses of 2, 5, and 12b-PPh3 are reported.
中文翻译:

[(tmeda)Ni(CH 3)2 ],同位素标记的[(tmeda)Ni(13 CH 3)2 ]和中性螯合镍甲基络合物的实用合成
基于市售的甲基锂和[(tmeda)Ni(acac),合成N,N,N ' ,N'-四甲基乙二胺镍二甲基,[(tmeda)Ni(CH 3)2 ](5)的实用程序)2 ](1)(acac =乙酰丙酮酸),[(tmeda)Ni(OAc)2 ](2)(OAc =乙酸盐),[(tmeda)NiSO 4 ](3)或[(tmeda)NiF 2 ] (4)已开发。以2为起始原料,可重现> 14 g(约68 mmol,85%)的5单批反应是可行的。这种合成途径还可以通过中间分离[[tmeda)2–3从13 CH 3 I开始形成和分离13 C标记的[(tmeda)Ni(13 CH 3)2 ](5- 13 CH 3)。(Li 13 CH 3)4 ] x(10)。的碘化物杂质的量10推回碘化物催化分解5- 13 CH 3乙烷和镍黑色由熊田型偶联是成功的隔离必不可少5- 13 CH 3。与[(tmeda)Ni(acac)2 ](1)相比,[(tmeda)Ni(OAc)2 ](2)是优于5的镍前体,因为(a)从硅中去除了乙酸锂(9)。粗反应混合物比除去的乙酰丙酮化锂和(b)κ容易得多2 - N,O -salicylaldiminato镍甲基络合物(12)或κ 2 -P,O- -phosphinesulfonato镍甲基络合物(14)可以很容易地通过一锅法分别从2,甲基锂和水杨醛亚胺(11)或phospho磺酸盐(13)开始制备。的X射线衍射分析2,5,和图12B-PPH 3中报告。
更新日期:2020-09-28
中文翻译:

[(tmeda)Ni(CH 3)2 ],同位素标记的[(tmeda)Ni(13 CH 3)2 ]和中性螯合镍甲基络合物的实用合成
基于市售的甲基锂和[(tmeda)Ni(acac),合成N,N,N ' ,N'-四甲基乙二胺镍二甲基,[(tmeda)Ni(CH 3)2 ](5)的实用程序)2 ](1)(acac =乙酰丙酮酸),[(tmeda)Ni(OAc)2 ](2)(OAc =乙酸盐),[(tmeda)NiSO 4 ](3)或[(tmeda)NiF 2 ] (4)已开发。以2为起始原料,可重现> 14 g(约68 mmol,85%)的5单批反应是可行的。这种合成途径还可以通过中间分离[[tmeda)2–3从13 CH 3 I开始形成和分离13 C标记的[(tmeda)Ni(13 CH 3)2 ](5- 13 CH 3)。(Li 13 CH 3)4 ] x(10)。的碘化物杂质的量10推回碘化物催化分解5- 13 CH 3乙烷和镍黑色由熊田型偶联是成功的隔离必不可少5- 13 CH 3。与[(tmeda)Ni(acac)2 ](1)相比,[(tmeda)Ni(OAc)2 ](2)是优于5的镍前体,因为(a)从硅中去除了乙酸锂(9)。粗反应混合物比除去的乙酰丙酮化锂和(b)κ容易得多2 - N,O -salicylaldiminato镍甲基络合物(12)或κ 2 -P,O- -phosphinesulfonato镍甲基络合物(14)可以很容易地通过一锅法分别从2,甲基锂和水杨醛亚胺(11)或phospho磺酸盐(13)开始制备。的X射线衍射分析2,5,和图12B-PPH 3中报告。



























 京公网安备 11010802027423号
京公网安备 11010802027423号