当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
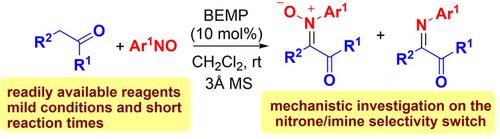
Nitrone/Imine Selectivity Switch in Base‐Catalysed Reaction of Aryl Acetic Acid Esters with Nitrosoarenes: Joint Experimental and Computational Study
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-09-16 , DOI: 10.1002/adsc.202000855 Chiara Volpe 1 , Sara Meninno 1 , Angelo Roselli 1 , Michele Mancinelli 2 , Andrea Mazzanti 2 , Alessandra Lattanzi 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-09-16 , DOI: 10.1002/adsc.202000855 Chiara Volpe 1 , Sara Meninno 1 , Angelo Roselli 1 , Michele Mancinelli 2 , Andrea Mazzanti 2 , Alessandra Lattanzi 1
Affiliation
|
Herein we report a mild and diastereoselective access to ketonitrones by reacting easily available aryl acetic acid esters and other active methylene compounds, with nitrosoarenes under catalytic loading of 2‐tert‐butylimino‐2‐diethylamino‐1,3‐dimethylperhydro‐1,3,2‐diazaphosphorine (BEMP) at room temperature. Depending on the substitution pattern and nature of the aryl moiety, a switch toward the formation of imines can be observed. The mechanistic framework is put to scrutiny by experimental and theoretical studies, pointing to the formation of a nitroso aldol intermediate, whose fate toward one of the competing pathways, namely hydride transfer or elimination, would depend upon the NOH/CHα relative acidities.
中文翻译:

芳基乙酸酯与亚硝基芳烃的碱催化反应中的氮/亚胺选择性开关:联合实验和计算研究
在此,我们报告了在2-叔丁基亚氨基-2-二乙基氨基-1,3-二甲基过氢-1,3,室温下使用2-重氮磷(BEMP)。取决于取代模式和芳基部分的性质,可以观察到向亚胺形成的转换。在机械框架由实验和理论研究投入推敲,指向形成亚硝基羟醛中间体,其命运朝向竞争性途径,即氢化物转移或消除一个,将取决于NOH / CH α相对酸度。
更新日期:2020-09-16
中文翻译:

芳基乙酸酯与亚硝基芳烃的碱催化反应中的氮/亚胺选择性开关:联合实验和计算研究
在此,我们报告了在2-叔丁基亚氨基-2-二乙基氨基-1,3-二甲基过氢-1,3,室温下使用2-重氮磷(BEMP)。取决于取代模式和芳基部分的性质,可以观察到向亚胺形成的转换。在机械框架由实验和理论研究投入推敲,指向形成亚硝基羟醛中间体,其命运朝向竞争性途径,即氢化物转移或消除一个,将取决于NOH / CH α相对酸度。

























 京公网安备 11010802027423号
京公网安备 11010802027423号