Journal of Proteomics ( IF 3.3 ) Pub Date : 2020-09-17 , DOI: 10.1016/j.jprot.2020.103987 Luitzen de Jong 1 , Winfried Roseboom 1 , Gertjan Kramer 1
|
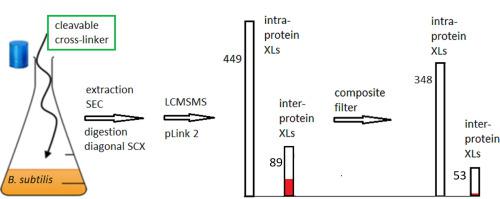
In vivo chemical cross-linking combined with LCMSMS of digested extracts (in vivo CX-MS) can reveal stable and dynamic protein-protein interactions at proteome-wide scale and at peptide level. In vivo CX-MS requires a membrane permeable and cleavable cross-linker and a fast and sensitive search engine to identify the linked peptides. Here we explore the use of the search engine pLink 2 to identify cross-links induced in exponentially growing Bacillus subtilis cells treated in culture with bis(succinimidyl)-3-azidomethyl-glutarate (BAMG). Cross-linked peptide pairs were identified by pLink 2 in very short time at an overall FDR of <5%. To also obtain a FDR <5% for non-redundant inter-protein cross-linked peptide pairs additional threshold values were applied for matched fragment intensity and for the numbers of unambiguous y and b ions assigned to both composite peptides. Also the mass- and charge-dependent retention times of target peptides purified by diagonal strong cation exchange chromatography were used as a criterion to distinguish true from false positives. After application of the composite filter new protein-protein interactions were revealed among others between the global transcriptional repressor AbrB and elongation factor Tu and between the essential protein YlaN of unknown function and the ferric uptake repressor Fur.
Significance
Important for reliable identification of PPIs by chemical cross-linking in vivo is a low FDR of non-redundant inter-protein peptide pairs. Here we describe how to recognize the presence of spurious interactions in a dataset of cross-linked peptide pairs enriched by 2D strong cation exchange chromatography and identified by LCMSMS by taking into account chromatographic behavior of cross-linked peptide pairs and protein abundance of corresponding peptides. Based on these criteria we assessed that the FDR of the fraction of non-redundant inter-protein cross-linked peptide pairs was approx. 20–25% by interrogating an entire species specific database at an overall FDR of 5% or 0.1% with a search engine that otherwise scores best in sensitivity among other search engines. We have defined a composite filter to decrease this high FDR of inter-protein cross-linked peptide pairs to only about 2%.
中文翻译:

通过体内交联检测到的低FDR的蛋白质-蛋白质相互作用的复合过滤器。
体内化学交联与消化提取物的LCMSMS结合(体内CX-MS)可以在蛋白质组范围内和肽水平上揭示稳定和动态的蛋白质-蛋白质相互作用。体内CX-MS需要膜可渗透和可裂解的交联剂,以及快速灵敏的搜索引擎来识别连接的肽。在这里,我们探索使用搜索引擎pLink 2来识别在指数增长的枯草芽孢杆菌中诱导的交叉链接双(琥珀酰亚胺基)-3-叠氮基甲基戊二酸酯(BAMG)培养的细胞。通过pLink 2在很短的时间内以小于5%的总FDR鉴定了交联的肽对。为了也获得非冗余的蛋白间交联肽对的FDR <5%,对匹配的片段强度以及分配给两种复合肽的y和b离子明确的数量应用了其他阈值。通过对角强阳离子交换色谱纯化的目标肽的质量和电荷依赖性保留时间也用作区分真假阳性的标准。
意义
通过体内化学交联可靠鉴定PPI的重要因素是非冗余蛋白间肽对的低FDR。在这里,我们描述了如何通过考虑到交联肽对的色谱行为和相应肽的蛋白质丰度,来识别通过2D强阳离子交换色谱富集并通过LCMSMS鉴定的交联肽对数据集中的虚假相互作用。基于这些标准,我们评估了非冗余蛋白间交联肽对部分的FDR约为。通过使用搜索引擎以5%或0.1%的整体FDR询问整个物种特定的数据库,从而获得20-25%的搜索结果,否则该搜索引擎在其他搜索引擎中的灵敏度最高。


























 京公网安备 11010802027423号
京公网安备 11010802027423号