Chemistry and Physics of Lipids ( IF 3.4 ) Pub Date : 2020-09-17 , DOI: 10.1016/j.chemphyslip.2020.104975 Kenneth M F Miasaki 1 , Natalia Wilke 2 , João Ruggiero Neto 1 , Dayane S Alvares 1
|
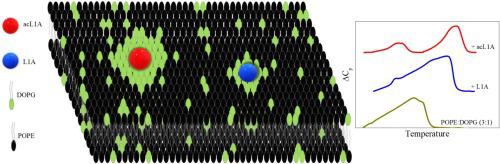
The synthetic peptides L1A and its acetylated analog (acL1A) display potent Gram-negative bactericidal activities without being hemolytic. We have gathered evidence that the N-terminal acetylation of L1A enhances the lytic activity in anionic vesicles with high capability to insert into and disturb lipid packing of model membranes. Here, the impact of L1A and acL1A was evaluated on a model membrane that mimics the cytoplasmic membrane of Gram-negative bacteria, which is rich in phosphatidylethanolamine (PE) and phosphatidylglycerol (PG), using 3:1 mixture of POPE/DOPG and a variety of techniques. We followed peptide adsorption and penetration by zeta potential determination of large unilamellar vesicles, accessibility of tryptophan residue to acrylamide by quenching assays, and Gibbs isotherms. The secondary structure of the peptide on the membranes was assessed using circular dichroism. Peptide mixing ability with the lipids and phase segregation was assessed by the observation of Langmuir monolayers with fluorescence microscopy, as well as with differential scanning calorimetry thermograms of multilamellar vesicles. All in all, the results indicate that both peptides adsorb and penetrate POPE/DOPG membranes with similar affinities, decreasing the surface charge, and adopting alpha structures. Both peptides mix with DOPG and demix from POPE, and consequently, persist at the interface to larger surface pressures in the presence of PG than in pure PE monolayers. This selective degree of mixing of the peptides with PE and PG leads to peptide-induced segregation of PG from PE, being the less charged peptide, acL1A, able to segregate the lipids more efficiently.
中文翻译:

乳脂素样肽的N末端乙酰化可增强模型膜中PE / PG的分离。
合成肽L1A及其乙酰化类似物(acL1A)表现出有效的革兰氏阴性杀菌活性,而不会溶血。我们已经收集了证据,表明L1A的N端乙酰化增强了阴离子小泡囊的裂解活性,并具有插入和破坏模型膜脂质堆积的高能力。在这里,使用POPE / DOPG和一种3:1的混合物,在模拟革兰氏阴性细菌胞质膜的模型膜上评估了L1A和acL1A的影响,该革兰氏阴性细菌富含磷脂酰乙醇胺(PE)和磷脂酰甘油(PG)。各种技术。我们通过zeta电位测定大型单层囊泡,色氨酸残基通过淬灭测定法和丙烯酰胺等温线对丙烯酰胺的可及性来跟踪肽的吸附和渗透。使用圆二色性评估膜上肽的二级结构。通过用荧光显微镜以及多层囊泡的差示扫描量热法热分析法观察Langmuir单层膜来评估肽与脂质的混合能力和相分离。总而言之,结果表明两种肽均以相似的亲和力吸附并穿透POPE / DOPG膜,从而降低了表面电荷并采用了α结构。两种肽都与DOPG混合并从POPE中分离,因此,在存在PG的情况下,与纯PE单层相比,它们在界面处持续存在更大的表面压力。肽与PE和PG的这种选择性混合会导致肽诱导的PG与PE的分离,这是电荷较低的肽acL1A,



























 京公网安备 11010802027423号
京公网安备 11010802027423号