当前位置:
X-MOL 学术
›
Soft Matter
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Lyotropic liquid crystal phase behavior of a cationic amphiphile in aqueous and non-stoichiometric protic ionic liquid mixtures.
Soft Matter ( IF 3.4 ) Pub Date : 2020-09-15 , DOI: 10.1039/d0sm01298j Dilek Yalcin 1 , Calum J Drummond 1 , Tamar L Greaves 1
Soft Matter ( IF 3.4 ) Pub Date : 2020-09-15 , DOI: 10.1039/d0sm01298j Dilek Yalcin 1 , Calum J Drummond 1 , Tamar L Greaves 1
Affiliation
|
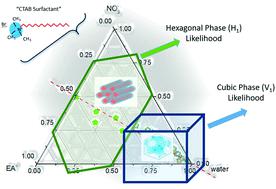
Protic ionic liquids (PILs) are the largest and most tailorable known class of non-aqueous solvents which possess the ability to support amphiphile self-assembly. However, little is known about the effect of solvent additives on this ability. In this study, the lyotropic liquid crystal phase (LLCP) behavior of the cationic surfactant cetyltrimethylammonium bromide (CTAB) was investigated in the model PILs of ethylammonium nitrate (EAN) and ethanolammonium nitrate (EtAN), and derived multi-component solvent systems containing them to determine phase formation and diversity with changing solvent composition. The solvent systems were composed of water, nitric acid and ethylamine (or ethanolamine), with 26 unique compositions for each PIL covering the apparent pH and ionicity ranges of 0–13.5 and 0–11 M, respectively. The LLCPs were studied using cross polarized optical microscopy (CPOM) and small and wide-angle X-ray scattering (SAXS/WAXS). Partial phase diagrams were constructed for CTAB concentrations of 50 wt% and 70 wt% in the temperature range of 25 °C to 75 °C to characterise the effect of surfactant concentration and temperature on the LLCPs in each solvent environment. Normal micellar (L1), hexagonal (H1) and bicontinuous cubic (V1) phases were identified at both surfactant concentrations, and from temperatures as low as 35 °C, with large variations dependent on the solvent composition. The thermal stability and diversity of phases were greater and broader in solvent compositions with excess precursor amines present compared to those in the neat PILs. In acid-rich solvent combinations, the same phase diversity was found, though with reduced onset temperatures of phase formation; however, some structural changes were observed which were attributed to oxidation/decomposition of CTAB in a nitric acid environment. This study showed that the ability of PIL solutions to support amphiphile self-assembly can readily be tuned, and that the ability of PILs to promote amphiphile self-assembly is robust, even with other solvent species present.
中文翻译:

阳离子两亲物在水性和非化学计量质子离子液体混合物中的溶致液晶相行为。
质子离子液体(PIL)是已知的最大和最可定制的一类非水溶剂,具有支持两亲物自组装的能力。然而,关于溶剂添加剂对该能力的影响知之甚少。在这项研究中,在硝酸乙基铵(EAN)和乙醇硝酸铵(EtAN)的模型PIL中研究了阳离子表面活性剂十六烷基三甲基溴化铵(CTAB)的溶致液晶相(LLCP)行为,并推导了包含它们的多组分溶剂体系确定随着溶剂组成的变化而形成的相和多样性。溶剂系统由水,硝酸和乙胺(或乙醇胺)组成,每种PIL具有26种独特的成分,分别覆盖表观pH值和离子性范围为0–13.5和0–11M。使用交叉偏振光学显微镜(CPOM)和小和广角X射线散射(SAXS / WAXS)研究了LLCP。在25°C至75°C的温度范围内,CTAB浓度分别为50 wt%和70 wt%时,绘制了部分相图,以表征每种溶剂环境中表面活性剂浓度和温度对LLCP的影响。正常胶束(L1),六边形(H 1)和双连续立方(V 1在两种表面活性剂浓度下以及从低至35°C的温度下均已鉴定到)相,其变化取决于溶剂组成。与纯PIL中的胺相比,存在过量前体胺的溶剂组合物中的热稳定性和相的多样性更大,范围更广。在富酸的溶剂组合中,尽管相形成的起始温度降低,但发现了相同的相多样性。然而,观察到一些结构变化,这归因于在硝酸环境中CTAB的氧化/分解。这项研究表明,可以很容易地调节PIL溶液支持两亲物自组装的能力,并且即使存在其他溶剂种类,PIL促进两亲物自组装的能力也很强。
更新日期:2020-09-23
中文翻译:

阳离子两亲物在水性和非化学计量质子离子液体混合物中的溶致液晶相行为。
质子离子液体(PIL)是已知的最大和最可定制的一类非水溶剂,具有支持两亲物自组装的能力。然而,关于溶剂添加剂对该能力的影响知之甚少。在这项研究中,在硝酸乙基铵(EAN)和乙醇硝酸铵(EtAN)的模型PIL中研究了阳离子表面活性剂十六烷基三甲基溴化铵(CTAB)的溶致液晶相(LLCP)行为,并推导了包含它们的多组分溶剂体系确定随着溶剂组成的变化而形成的相和多样性。溶剂系统由水,硝酸和乙胺(或乙醇胺)组成,每种PIL具有26种独特的成分,分别覆盖表观pH值和离子性范围为0–13.5和0–11M。使用交叉偏振光学显微镜(CPOM)和小和广角X射线散射(SAXS / WAXS)研究了LLCP。在25°C至75°C的温度范围内,CTAB浓度分别为50 wt%和70 wt%时,绘制了部分相图,以表征每种溶剂环境中表面活性剂浓度和温度对LLCP的影响。正常胶束(L1),六边形(H 1)和双连续立方(V 1在两种表面活性剂浓度下以及从低至35°C的温度下均已鉴定到)相,其变化取决于溶剂组成。与纯PIL中的胺相比,存在过量前体胺的溶剂组合物中的热稳定性和相的多样性更大,范围更广。在富酸的溶剂组合中,尽管相形成的起始温度降低,但发现了相同的相多样性。然而,观察到一些结构变化,这归因于在硝酸环境中CTAB的氧化/分解。这项研究表明,可以很容易地调节PIL溶液支持两亲物自组装的能力,并且即使存在其他溶剂种类,PIL促进两亲物自组装的能力也很强。


























 京公网安备 11010802027423号
京公网安备 11010802027423号